1. Introduction
Epoxy resin-based composites are often employed in a variety of civil and industrial applications thanks to their superior mechanical, electrical insulation, bonding, and process flexibility qualities. The Antarctic continent has 70% of the freshwater resources on earth and the world's largest reserves of oil, natural gas and various mineral resources. With the increasing depletion of the earth's resources, the development of the Antarctic continent will become a research hotspot for all countries. The polar region has special low-temperature environmental conditions, the average annual temperature of Antarctica is -50℃, the lowest temperature is -89.2℃, which brings challenges to ship equipment, marine engineering equipment, coastal construction and construction projects and machinery maintenance. The embrittlement of epoxy resin composites under low-temperature conditions is one of the key issues to be addressed.
Therefore, this paper focuses on the research problem of toughening epoxy resin-based composites in a low-temperature environment, and investigates and summarizes various toughening methods and future development directions of previous research, which provides theoretical guidance for the application of epoxy resin-based composites in a low-temperature environment.
2. Epoxy resin and its application
Epoxy resin is a polymer zwitterion containing two or more epoxy groups, mainly organic compounds such as aromatic hydrocarbons, capable of obtaining thermosets through the ring-opening reaction of epoxy groups. Epoxy resin [1] has the following advantages: (1) high bonding strength and wide bonding surface. When epoxy resin cures, the polar groups with high activity make the resin molecules react chemically with the interface, and the resin cures to form a three-dimensional net-like cross-linked structure, plus the molecules themselves have a certain cohesive force, which makes the cured products have a high bonding strength. Moreover, epoxy resin has good bonding with most metals and non-metals, and its bonding strength with many non-metal materials often exceeds the strength of the material itself, thus epoxy resin is often used as the matrix of composite materials. (2) Small curing shrinkage, epoxy resin has no small molecules in the curing reaction, so the curing shrinkage is very low, only about 1%-2%. Therefore, the epoxy resin products are dimensionally stable and not easy to be damaged by cracking in actual use. (3) High stability, no deterioration-prone components, long service life, cured products are dense three-dimensional network, with good chemical stability. (4) High mechanical strength, the cured epoxy resin has a dense three-dimensional cross-linked structure and strong cohesion, so its mechanical strength is high. (5) Excellent electrical insulation, after curing, epoxy resin has dense structure, no more free ions, and low water absorption, so it has good electrical insulation.
Epoxy resin and its composite materials have the following applications [2]: (1) Coating field: epoxy resin can have good adhesion, corrosion resistance and toughness, and can be used to make solvent, non-solvent, powder and water-based coatings, which are widely used in anti-corrosion, naval coatings, electrical insulation and other fields. (2) Adhesives: Except for non-polar plastics such as olefins, epoxy resin can bond with various metals and non-metal materials. (3) For electronic packaging and electrical materials: epoxy resin has good electrical insulation, heat resistance, dielectric properties and sealing properties, and is widely used in the insulation and packaging of electronic devices and circuit boards, and can be added to ceramics to enhance its thermal conductivity. (4) Preparation of engineering plastics and composite materials, epoxy resin has good adhesion and mechanical properties, plus epoxy resin can be modified to obtain better performance, often used as the matrix of composite materials.
3. Research progress of epoxy resin low temperature performance and low temperature toughening
3.1. Low-temperature performance of epoxy resins
Research on the low-temperature properties and theory of epoxy resins has started since the 1960s. In order to optimize the epoxy system for use in low-temperature engineering, Ueki T. et al. [3] designed a number of different epoxy systems and tested their low-temperature mechanical and thermal properties. The results indicated that the network structure should be preferred over the chemical structure in order to increase low-temperature fracture toughness. To increase the fracture toughness of bisphenol A epoxy resin systems at low temperatures, Sawa F. et al. [4] combined two epoxy resins with variable quantities of epoxy groups and utilized polyfunctional epoxy tetrakis (glycidylmethyl) diamine (TGMXDA) as a modifier. According to the findings, resins with greater epoxy equivalents and flexible molecular architectures have higher fracture toughness at low temperatures. They also learned [5] that the fracture toughness of epoxy resins is influenced by the intermolecular forces and tension relaxation at the crack tip. The crack tip stress relaxation occurs at low temperatures in epoxy resins with higher cross-linked molecular weights, demonstrating the significance of these two factors in enhancing fracture toughness (Figure 1).
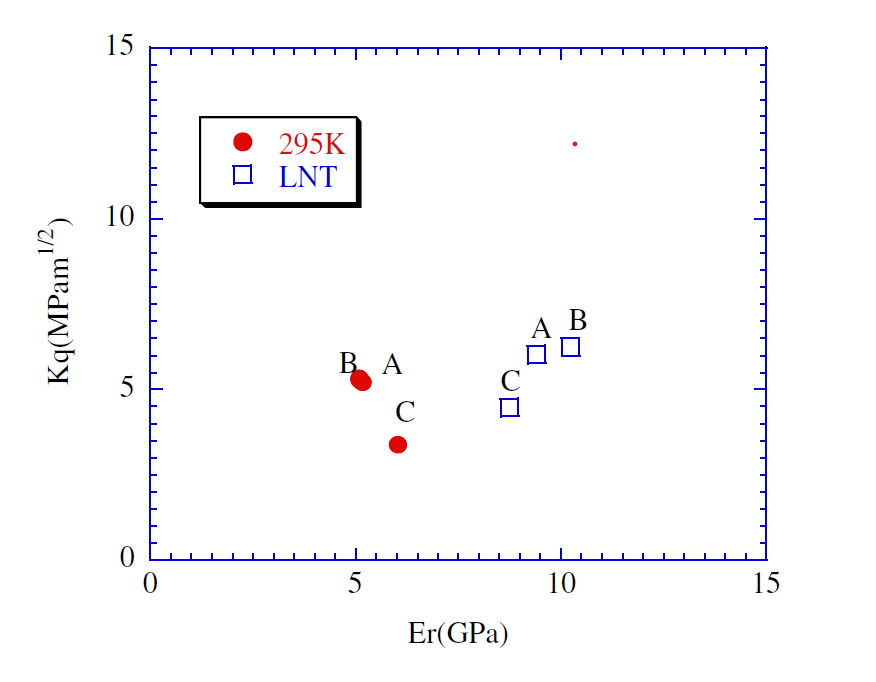
Figure 1. Kq and Young's modulus Er are related [4]
Free volume is an intrinsic factor that determines the absorption and diffusion of small molecules within the polymer. In three-dimensional epoxy networks, the free volume originates from those cavities formed due to the irregular stacking of the polymer network. Therefore, the free volume is affected by the cross-link density and network structure. As the temperature decreases, the free volume of the three-dimensional epoxy resin cross-linked network decreases, and below the secondary transition temperature, the epoxy resin chain segments are frozen and basically lose their motility. In the atmosphere of low temperature, the three-dimensional epoxy resin cross-linked network's free volume drops, which makes the chain segment less motile and leads to the stress concentration inside the chain segment and fracture quickly when it is subjected to an external force. Therefore, enhancing epoxy's toughness at low temperatures can be accomplished by managing the free volume of the three-dimensional cross-linked network of epoxy resin. Increasing the free volume at low temperatures can reduce stress in the epoxy resin and enhance the motility of individual molecules in the chain, which increases the fracture toughness of the epoxy resin.
3.2. Research progress on low temperature toughening of epoxy resins
3.2.1. Toughening of rubber elastomers. The current methods to improve the toughness of epoxy resins are mainly physical and chemical modifications [6]. The physical modification does not change the molecular structure of the original cross-linked network of the epoxy resin and is modified by adding a second phase such as rubber, thermoplastic resin, liquid crystal polymer and nanoparticles, which are co-blended with the epoxy resin. Through copolymerization reactions, chemical changes graft or embed flexible chain segments into the epoxy resin's three-dimensional network structure.
To enhance the low-temperature mechanical characteristics of epoxy resins, Zhao Y. [7] et al. mixed epoxy resins with carboxylated nitrile nanorubber (NR). The results showed that the epoxy blends containing 15phrNR at 77K increased the tensile strength by 40.2%, the fracture toughness (KIC) by 48.3%, and Young's modulus was slightly reduced due to the introduction of flexible NR. Impact experiments on epoxy resins toughened with CSR particles were carried out by Brown H. R. et al. [8] at 100K and room temperature. They discovered that CSR particles peeled off at the core-shell interface in front of the crack tip.Shell interface in front of the crack tip and debonded between the shell and epoxy resin tested at 100K. The investigation came to the conclusion that the major processes for toughening the CSR-modified epoxy resin at room temperature are enhanced formation of many voids and localized shear bands. The increase in secondary cracking and the interaction of nearby residual stress fields are the two basic mechanisms through which CSR rubber particles can toughen epoxy resins at low temperatures (Figure 2).
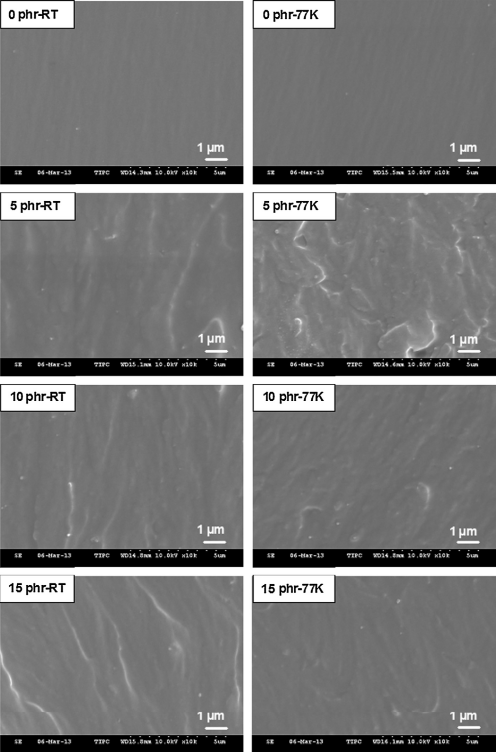
Figure 2. Enlarged SEM pictures of the NR-epoxy blends' fracture surfaces [7]
3.2.2. Toughening of thermoplastic resins. The low-temperature toughness of epoxy systems can be improved by co-blending modification of thermoplastic resins with epoxy resins. He Y.X. et al. [9] used three different types of thermoplastics, polycarbonate (PC), poly (etherimide) (PEI) and polybutylene terephthalate (PBT), to modify epoxy resins. According to the dynamic mechanical study, the energy storage modulus of epoxy resins treated with PEI, PC, and PBT rose by 30%, 21%, and 17%, respectively, when compared to epoxy resins that had not been changed.The results showed that the presence of thermoplastics could increase the impact strength of the epoxy resins and decrease the CTE of the epoxy matrix 1.5 wt.% PEI content of the epoxy resin increased the impact strength and decreased the CTE by 45% and 23%, respectively, at 77K compared to the pure epoxy resin. Therefore, PEI and PC can effectively prevent the formation of microcracks in carbon fiber/epoxy resin matrix composites during the low-temperature thermal cycling.
3.2.3. Toughening of nanomaterials. In domestic and international studies, nanomaterials such as nano-rubber particles, multi-walled carbon nanotubes and graphene are also often used to toughen epoxy resins, and some results have been achieved [10]. Multi-walled carbon nanotubes (MWCNTs) were introduced to bisphenol F epoxy resin by Chen Z. K. et al. [11] using an ultrasonic approach. The results showed that the system's low-temperature mechanical characteristics were optimum when 0.5% mass fraction of MWCNTs was added. When 0.5% of the mass of MWCNTs was added, the mechanical properties of the system were optimized. The tensile strength at 77 K, Young's modulus, elongation at break, and impact strength all increased in comparison to the pure epoxy resin, demonstrating that the modified epoxy resin's low-temperature toughness and strength were enhanced.Although nanomaterials have achieved some results in improving the low-temperature performance of epoxy resin, the preparation process of nanomaterials is complicated and the yield is low, which makes it difficult to be applied in large quantities in engineering.
3.2.4. Toughening of flexible chain segments. Flexible chain segments are frequently used to embed in dense epoxy resin networks to increase the low-temperature durability of epoxy resins because they retain some rotational mobility even at low temperatures without becoming entirely frozen [12]. Shang Chengyuan et al. [13] obtained epoxy resins containing flexible polyether side chains by reacting terminal isocyanate polyethers with epoxy resins and found that the impact toughness was improved by 34.1% and 51.9% over that of pure epoxy resin at room temperature and -60°C, respectively. Feng Q P et al. [14] modified the E54/methytetrahydrophthalic anhydride system with poly(ethylene glycol) (PEG-4000) by introducing polyethylene glycol into epoxy resin to improve low-temperature tensile strength, ductility and impact resistance. Flexible chain segments are frequently used to embed in dense epoxy resin networks to increase the low-temperature durability of epoxy resins because they retain some rotational mobility even at low temperatures without becoming entirely frozen.
3.2.5. Toughening of hyperbranched polymers. Epoxy resins' mechanical characteristics at room temperature can be significantly enhanced by hyperbranched polymers. Modified bisphenol by Yang, Jiao, et al. [15] at ambient temperature and liquid nitrogen temperature, experiments on the mechanical and thermal properties of an epoxy resin with hydroxyl-functionalized hyperbranched polyester H30 were conducted (77K). With an increase in H30 content, the low-temperature tensile strength exhibited a trend of increasing and subsequently declining. The hydrogen bond formed in the cured epoxy system was enhanced at 77k due to thermal shrinkage. The smaller free volume led to an increase in increased intermolecular forces, resulting in higher tensile and impact strengths at low temperatures. the H30 toughening mechanism can be explained by the in situ enhanced toughening mechanism.
4. Conclusion
Toughening modification has broadened the scope of industrial applications of epoxy resins, and to a certain extent effectively improved the quality of brittle epoxy resins. With the development of composite material preparation technology, the performance requirements of epoxy resins are bound to become higher and higher, and the research of epoxy resin toughening can be promoted from the following aspects in the future.
(1) Toughening mechanisms mainly include cavity expansion, crack riveting, crack nailing, crack deflection, stress concentration reduction, cross-linked network structure formation, etc. At the same time, new toughening mechanisms and methods are explored and discovered to promote the application and development of toughened epoxy resin materials.
(2) Modify epoxy resins from multiple angles by combining nanostructures with flexible chain segments, rubber particles with hyperbranched polymers, single-component filling or multi-component filling, etc. While giving the material good mechanical properties, more functionalities are added, such as flame retardancy and wear resistance.
(3) Research in terms of operational implementability and other aspects to avoid wasting resources and make better use of the excellent properties of epoxy resins. Epoxy resin toughening is still difficult to develop in large-scale industrial production, mainly due to issues such as design of process parameters and cost control, which need to be urgently addressed by researchers.
Acknowledgement
There are so many people I need to thank in my school career, the most important one is my parents' patient education that has enabled me to maintain a humble and studious attitude, and secondly, I would like to thank my mentors professor Erik Luijten, professor Tan and all the teachers who have supported and encouraged me for bringing me to the door of research.



