1. Introduction
1.1. Background introduction
Retrosynthesis [1] is the approach by which we disintegrate a molecule into simpler components. It is highly valued in organic synthesis since it enables us to design possible synthetic routes for molecules that have never been synthesized before. This concept is not introduced in high school courses. However, for high school students, learning retrosynthesis is very helpful since it gives a deeper understanding in the reactivity and structure of molecules. Therefore, this work aims to give a guiding overview of retrosynthesis to young scholars based on principles of retrosynthesis and some strategies. This is done by summarizing given works of retrosynthesis and devising retrosynthesis examples for illustration.
1.2. Key concepts
Retrosynthesis focuses on disconnection—breaking bonds. When a bond is being disconnected, the target molecule (The molecule that we are trying to deconstruct, often known as TM) is broken into simpler synthons (The fragments produced by disconnection). Since synthons are often unstable (They are usually ions), synthetic equivalents are usually used to represent them. These are the more stable form of the synthons. Below listed the principles and some strategies of retrosynthesis, these are used to make sure a disconnection is effective.
2. Principles of retrosynthesis
2.1. Characteristics of a good disconnection [2]
Not all disconnections are effective. Certain principles are followed when trying to break a bond effectively.
1: The feasibility of forward reaction: Above all, when considering retrosynthesis (the backward reaction), it is crucial to think about whether the forward reaction is feasible. Therefore, most rules that determine whether a forward reaction will happen applies to retrosynthetic analysis. This includes the concepts of acidity (pKa), regioselectivity, stereochemistry, the reactivity of different functional groups, etc.
2: Reasonable mechanism: A good mechanism will ensure that the forward reaction is likely to happen.
3: Greatest simplification: Chemists will often focus on the most complicated part of the molecule, such as breaking a ring, a bridge or a long chain of molecules. Even though not all steps are simplifying, breaking the most complicated parts of a molecule is still regarded as the main focus of a retrosynthesis.
4: Giving recognizable products: Obtainable starting materials are required for a forward synthesis.
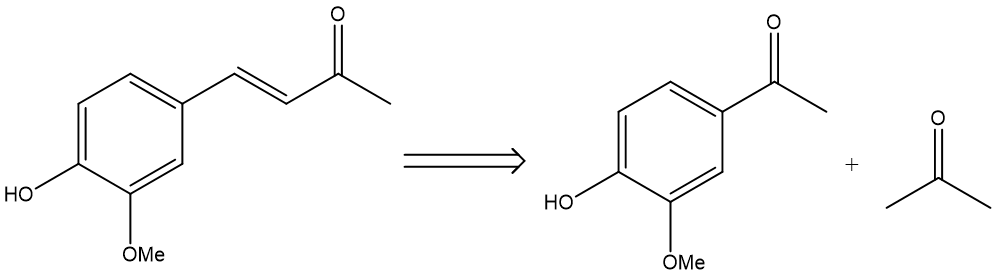
Here is an example of a good disconnection.
|
Figure 1. example of a good disconnection. |
In this case, the forward reaction would be an aldol condensation—The double bond is broken to form two carbonyls (the symbol is used to show the bond being disconnected). The reaction also gives recognizable products—vanillin and acetone respectively [3], and both of them are readily available. Thus, this disconnection is considered a good one.
2.2. Types of disconnections
One-group disconnection [4]: Disconnection focusing on one functional group only. This kind of disconnection focuses on alcohols, olefins, carbonyls, carboxylic acids and their derivatives. The basic rule for each of the categories are listed below. (Still, different substituent groups or different functional group derivatives can lead to completely different products.)
For simple alcohols: Generally speaking, it can be seen as the product of the reaction between a Grignard reagent or alkyl lithium and a carbonyl. The forward mechanism would therefore be the nucleophilic addition of a Grignard reagent on carbonyl groups.
For simple olefins: Olefins are made from the elimination of an alcohol. Therefore, chemists often treat an olefin as carbon chain with one of the carbons substituted by a hydroxy group. However, the Markovnikov’s rule should be considered when conducting the forward reaction, in order to make sure it gives the right product.
For ketones: Since ketone can be prepared by the oxidation of an alcohol, it can similarly be treated as the product of addition between a Grignard reagent and an aldehyde.
For acids: Similarly, acid can be seen as the product of the reaction between a Grignard reagent and a carbon dioxide.
For acid derivatives: This includes acyl chlorides, acid anhydrides, esters and amides, arranged by a decrease in reactivity. The acyl chlorides are prepared under treatment of acids with phosphorus pentachloride or thionyl chloride, and the functional group interconversion between acyl chlorides and other acid derivatives can be done under treatment with nucleophiles. Therefore, they can all be converted to carboxylic acid and undergo subsequent disconnections mentioned before.
Two-group disconnection: Disconnection that involves two functional groups simultaneously. For this category, the most common type of disconnection is between two oxygen-connected groups, know as (1, y) Dioxygenation pattern. This is introduced in the Strategies part.
Pericyclic disconnection: Disconnection of a ring, such as the Diels-alder reaction.
Illogical disconnection: Disconnection which forms bonds instead of breaking bonds. (That’s why it is “illogical”)
Fine tuning: The small changes of functional groups, including the following strategies:
FGI—functional group interconversion, such as converting an alcohol into a carbonyl, converting an amine into a nitro group, etc.
FGR—functional group removal, such as removing a halogen atom from a carbon chain, breaking a methyl ester to form the corresponding acid, etc.
FGA—functional group addition, such as adding a double bond into a carbon chain.
3. Strategies of Retrosynthesis
Many strategies are developed for retrosynthesis as to give more direct starting materials. Two of the strategies, (1, y) Dioxygenation pattern and symmetry, are included here.
3.1. (1, y) Dioxygenation Pattern [5]
Dioxygenation pattern refers to the situation when two oxygen-connecting carbons exist within a molecule. It is useful because all dioxygenation patterns of the same sort can be disconnected into similar synthetic equivalents. The typical deoxygenation patterns are:
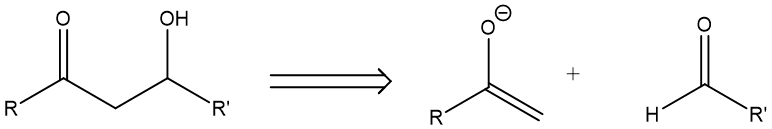
(1, 3) Dioxygenation Pattern: This is when two oxygen-connecting carbons are separated by one carbon in between. The prototypical pattern A in Fig.2 will then undergo a reverse of aldol reaction to give an aldehyde and an enolate anion.
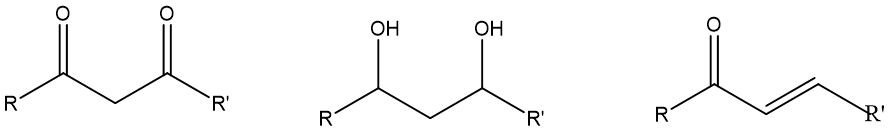
Note that for all dioxygenation patterns, other similar patterns can also be converted into the prototypical pattern using FGI. Fig.3 listed the structure B, C and D, which are similar patterns of dioxygenation. (For a double bond, one carbon has the same oxidation level as an alcohol, and the other has the oxidation level of an alkane. This is known as a “hidden” dioxygenation pattern)
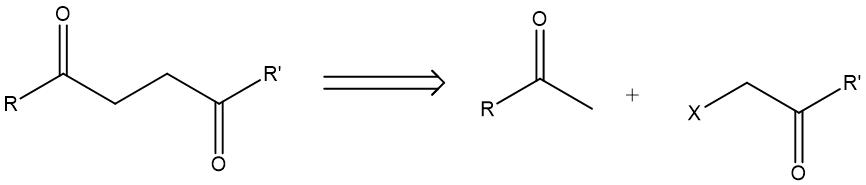
(1, 4) Dioxygenation pattern: This is when two oxygen-connecting carbons are separated by two carbons in between. A prototypical pattern looks like molecule E in Fig.4. Disconnecting E gives one methyl ketone and one α-halogenated ketone.
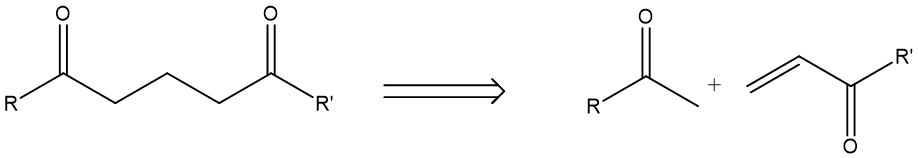
(1, 5) Dioxygenation pattern: Similarly, this is when two oxygen-connecting carbons are separated by three carbons in between. A prototypical pattern will look like molecule F in Fig.5. Disconnecting F gives the following synthetic equivalents, which is a reverse of Michael reaction.
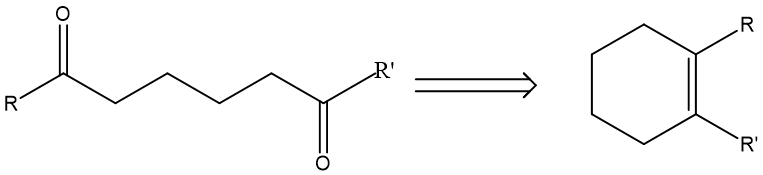
(1, 6) Dioxygenation pattern: This is when two oxygen-connecting carbons are separated by four carbons in between. However, the disconnection of (1, 6) Dioxygenation belongs to illogical disconnection, as it forms a bond to become a six-membered ring. A prototypical pattern looks like molecule G in Fig.6. In this case, ozonolysis is used to conduct the forward reaction.
A
Figure 2. (1, 3) Dioxygenation Pattern.
B C D
Figure 3. “hidden” dioxygenation pattern.
E
Figure 4. (1, 4) Dioxygenation pattern.
F
Figure 5. (1, 5) Dioxygenation pattern.
G
Figure 6. (1, 6) Dioxygenation pattern.
3.2. Convergent synthesis [4]
Convergent means that the synthetic route is arranged in parallel, instead of in linear. This is recommended because it increases the yield of the final product by reducing the number of steps for a synthesis. The idea of convergent synthesis is shown below:
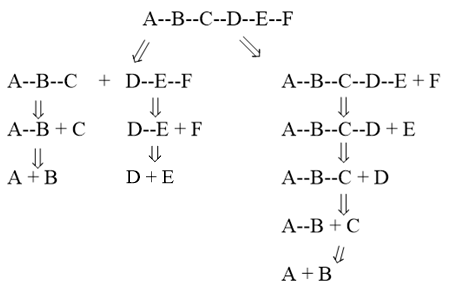
Figure 7. convergent synthesis.
The convergent synthesis of molecule A—B—C—D—E—F contains three steps in total (each chain of the synthetic route can be done separately). Assume that each step has a yield of 80%, then the yield of the final product would be 51.2%. However, for a linear synthetic route, five steps are required to get the final product, thus the yield of the final product would only be 32.8%. Therefore, chemists will often try to design a convergent synthetic route. This is done by breaking a complex molecule into two or more parts with similar complexity.
3.3. Symmetry
Again, from a practical standpoint, we may try to disconnect molecule into the synthetic equivalents which contain symmetrical geometry. This will increase the yield of the final product, and will also eliminate the impact of other functional groups. One example is shown in Applications of Retrosynthesis.
4. Applications of retrosynthesis
Example I: retrosynthesis using dioxygenation pattern and convergent synthesis [5]
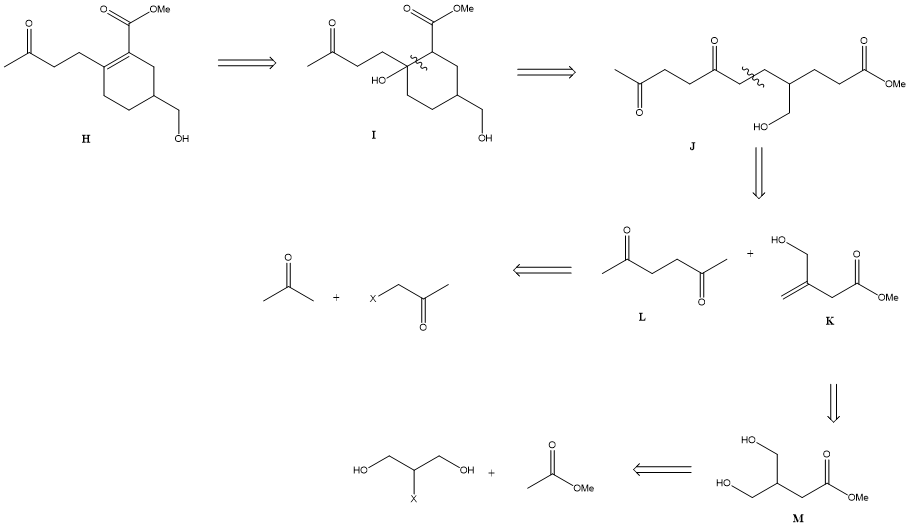
Figure 8. Example 1: retrosynthesis using dioxygenation pattern and convergent synthesis.
For this retrosynthesis above, molecule H has many deoxygenation patterns in it. Therefore, chemists will usually try each one of them and find the best way for disconnection. It turns out that when regarding the double bond as a carbon with an alcohol oxidation level (molecule I), breaking the (1, 3) dioxygenation pattern is a recommended choice [5], giving molecule J as the starting material. It is a good disconnection because the six-member ring is broken. When considering the disconnection of molecule J, breaking the (1, 5) dioxygenation patten is recommended, since it gives two synthetic equivalents of similar complexity, which refers to a convergent synthesis. Furthermore, molecule L has a symmetrical structure, making it easy to be further disconnected. When breaking molecule L, (1, 4) dioxygenation pattern is used, giving an acetone and an α-halogen acetone; When breaking molecule K, again the double bond is treated as a carbon connected to a hydroxy group (molecule M). Thus, (1, 4) dioxygenation is used to break molecule M into corresponding synthetic equivalents.
Example II: a comprehensive retrosynthesis [6]
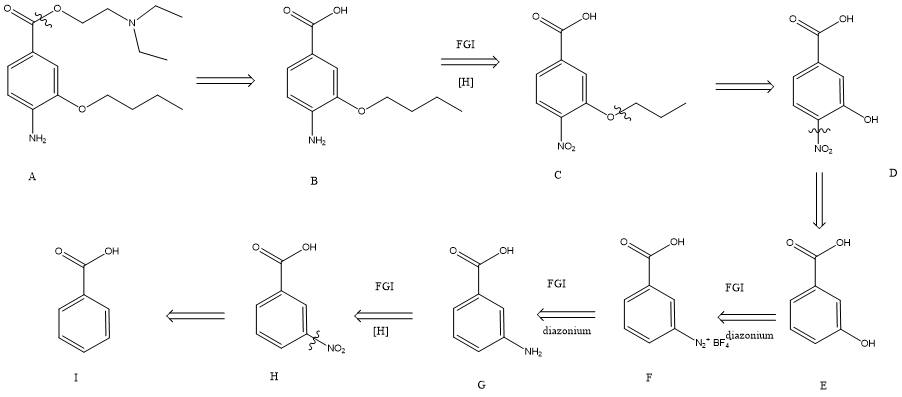
Figure 9. Example II: a typical comprehensive retrosynthesis.
For this example, it is necessary to consider the relative reactivity of each functional groups to make sure that things don’t mess up. Ester is the most reactive functional group in the molecule, so it is disconnected first, forming a carboxylic acid (molecule B) and an alcohol through ester hydrolysis. Then, the aim would be to break down the ether part, as it gives us the best possible simplification. However, amine will interfere with the reaction of ether through the alkylation of amine. Therefore, it is converted to a less reactive nitro group (molecule C). The next step would be the disconnection of C-O bonds (ester), forming compound D. In this case, the forward reaction could be the Williamson ether synthesis [7], forming compound D. Then, the C-N bond of the nitro group can be disconnected, giving a 3-hydroxybenzoic acid. Still, we can simplify the molecule further by dropping the hydroxy group to form a benzoic acid. In this case, FGI and diazonium ion [8] are used to convert the hydroxy group into a nitro group and remove it. Finally, molecule I is given as the starting material.
5. Conclusion
This work covers the key principles and strategies related to retrosynthesis analysis. The ideas are explained in a way that suits high school students with elementary skills in chemistry, which will give young scholars an idea of what a systematic retrosynthesis looks like. With the explanation and examples above, scholars will have a generalized understanding of retrosynthesis and be able to solve elementary retrosynthesis problems. This work will also be the basis for further retrosynthesis studies, which will help scientists to design reasonable total synthesis for important molecules.