1. Introduction
Colloidal gold is a colloidal solution composed of gold nanoparticles, which are gold atoms or clusters with diameters ranging from a few nanometers to tens of nanometers. Colloidal gold can be synthesized by various methods, such as chemical reduction, biological reduction, physical methods and microwave radiation. The most widely used technique is chemical reduction, which converts gold ions (Au3+) in chloroauric acid (HAuCl4) to gold atoms (Au0) by applying reducing agents such as sodium citrate, ascorbic acid, tannic acid, etc. By changing the reaction parameters, such as concentration, pH, temperature, stirring rate, etc., it is possible to alter the size, shape, and dispersion of the gold nanoparticles. The gold nanoparticles are usually negatively charged due to the adsorption of anions or ligands on their surface, which prevent them from aggregating and provide sites for further functionalization [1].
Colloidal gold is a hydrophobic colloid that maintains a stable colloidal system by electrostatic repulsion of negatively charged particles, which are dispersed and red in color, with diameters ranging from a few nanometers to tens of nanometers. Colloidal gold can be understood as the colloidal state of gold monomers. It is frequently utilized in biosensing, imaging, catalysis, and other sectors due to its remarkable optical features, including as localized surface plasmon resonance (LSPR). Colloidal gold is used as a tracer for antigen-antibody detection in the innovative immunolabeling method known as the colloidal gold technique. Colloidal gold was first discovered by Faraday in 1857, who prepared colloidal gold from chloroauric acid (HAuCl4) by reduction with reducing agents such as phosphorus, ascorbic acid, sodium citrate, tannic acid, etc., and discovered that the color of the colloidal gold could be changed from red to blue by adding a small amount of electrolyte to it, and finally coagulate into colorless, while adding gelatin or other macromolecules could prevent this change. His great discovery laid the scientific foundation for the preparation and application of colloidal gold. Using reducing chemicals, the gold element in chloroauric acid is reduced to create gold colloidal particles in the traditional method of making colloidal gold, which are negatively charged in an alkaline environment and can bind firmly to the positively charged groups of proteins, which can be used for subsequent testing, such as making colloidal gold test paper or card for the detection of substances.
This article will review the application, advantages and disadvantages of the colloidal gold technique [2].
2. Preparation method
2.1. Physical methods
The ultrasonic method uses the cavitation effect and high temperature and pressure of ultrasonic waves. And the metal or gold salt solution is dispersed into nanoscale gold particles. The laser irradiation method uses a laser beam to irradiate the metal or gold salt solution, producing local high temperature and pressure, making gold atoms or ions aggregate into nanoscale gold particles. The electrochemical method uses electrolytic current to electrolyze the metal or gold salt solution, making gold atoms or ions deposit at the cathode or anode to form nanoscale gold particles. The microwave irradiation method uses the electromagnetic field of a microwave to irradiate the metal or gold salt solution, producing local high temperature and pressure, making gold atoms or ions aggregate into nanoscale gold particles [3,4].
2.2. Chemical methods
There are many methods for preparing colloidal gold solutions, and the most commonly used is the chemical reduction method. The basic principle is to add a certain amount of reducing agent to a gold solution of a certain concentration to turn gold ions into gold atoms. Sodium citrate, tannic acid, ascorbic acid, white phosphorus, sodium borohydride, and others are frequently used reducing agents. Gold nanoparticles (AuNPs) have undergone a variety of production processes. These techniques involve reducing gold (III) chloride using mild reducing agents, as in Turkevich's citrate method's example, or utilizing organic solvents in Brust and Scriffin's two-phase synthesis technique. Among them, the Turkevich approach is acknowledged as the best option for producing GNPs in a green reagent due to its straightforward, one-step procedure, quick synthesis time, and support for a variety of reducing agents [5].
2.3. Biological methods
However, there are also some biological methods for preparing colloidal gold. For example, biological reducing agents such as bacteria, fungi, and plant extracts can be used to prepare colloidal gold. These biological reducing agents can reduce gold ions to gold nanoparticles, forming a colloidal gold solution. This process, which may be done at room temperature and doesn't require any chemical reducing agents, is regarded as a green synthesis approach [6].
3. Application
Colloidal gold can be used for various biomedical applications by modifying its surface with different molecules, such as antibodies, proteins, peptides, DNA, drugs, etc. These molecules can impart specific recognition, targeting, imaging or therapeutic functions to the gold nanoparticles. Immunochromatographic assay (ICA) is one of the most frequently used uses of colloidal gold, which is a rapid and simple detection method based on antigen-antibody specific binding reaction on a paper-based strip. Colloidal gold acts as a marker that binds to either the antibody or the antigen in the sample or on the strip. The binding results in a color change that can be visually observed at the test line or the control line. There are two main modes of ICA: sandwich mode and competitive mode. Sandwich mode is suitable for detecting large molecule antigens, such as proteins or viruses. In this mode, the sample antigen binds to both the antibody pre-coated on the test line and the antibody pre-bound with colloidal gold. The more antigen in the sample, the more color at the test line. Competitive mode is suitable for detecting small molecule antigens, such as hormones or drugs. In this mode, The colloidal gold-prebound antibody and the sample antigen that is pre-coated on the test line compete for binding to the antibody. The more antigen in the sample, the less color at the test line [7].
3.1. Application of disease detection
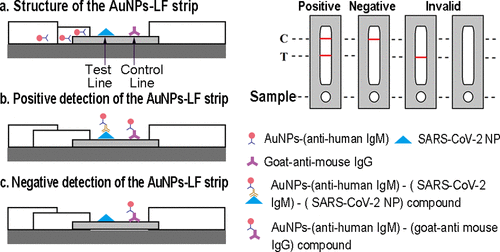
Colloidal gold technology for the detection of human or biological diseases can provide timely and effective information for disease diagnosis, prevention and treatment, which is of great significance for the protection of human and biological health. The technology can be used to detect infectious diseases such as COVID-19, dengue fever and epidemic hemorrhagic fever, as well as tumor markers, hormones and drugs. A common example is the colloidal gold method used to detect antibodies to the novel coronavirus. This method indirectly proves whether a person is infected with the novel coronavirus by detecting the presence of antibodies (such as IgG or IgM) that specifically bind to the virus (Figure 1). The side-flow detection method based on colloidal gold nanoparticles (AuNP-LF) has excellent sensitivity, good specificity, and ease of use, low cost, no need for instruments, etc., which is suitable for large-scale screening and early diagnosis. The detection time of the method was 15 minutes, the detection sensitivity was 0.5 μg/mL, and the detection specificity was 100% [8].
Figure 1. Colloidal Gold Nanoparticle-Based Lateral-Flow Assay for the Rapid Detection of IgM Antibodies Against the SARS-CoV2 Virus [8].
3.2. Application of food testing
Technology based on colloidal gold is used to identify dangerous ingredients in food. The protection of both human health and food safety is crucial. The technology can detect drug residues, pathogenic microorganisms, biotoxins, banned drugs and other harmful substances in food. A common example is the colloidal gold method used to detect aflatoxin B1 in food. This method determines whether food is contaminated by fungi by detecting the presence of antibodies that specifically bind to aflatoxin B1 (Figure 2). This method based on time-resolved fluorescence immunochromatography (TRFIA) is suitable for quick and sensitive quantitative analysis due to its high sensitivity, good specificity, ease of use, lack of labeling, and other benefits. This method's detection time was 10 minutes, the detection limit was 0.03 ng/mL, the detection linear range was 0.05 to 2.0 ng/mL, the recovery rate was 87.4% to 113.0%, and the relative standard deviation was 2.6% to 8.7% [9].
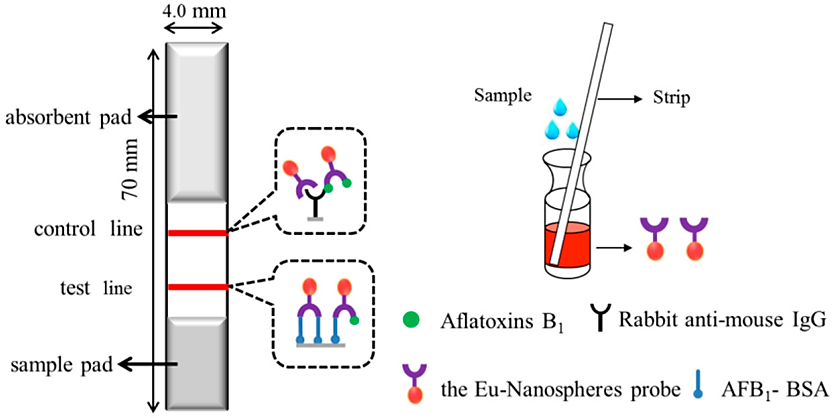
Figure 2. Schematic diagram of the determination of aflatoxins B1 in soybean paste by time-resolved fluorescence immunochromatography [9].
3.3. Application of environmental detection
The use of Colloidal gold technology in environmental detection is crucial to safeguarding both human health and environmental quality. The technology can detect pollutants such as organic matter, heavy metals and pathogenic bacteria in compost, or detect harmful substances such as pesticide residues, hormones and antibiotics in water quality. A common example is the detection of salmonella in compost by Colloidal gold technology, a common foodborne pathogen that is harmful to human and animal health and agricultural production (figure 3). The approach based on colloidal gold immunochromatography (CG-ICTS) is appropriate for quick and broad spectrum detection and has the advantages of high sensitivity, good specificity, simple operation, and no requirement for instrumentation. This method has a 15-minute detection window, a detection limit of 102 CFU/mL, a detection linear range of 102–107 CFU/mL, a recovery rate of 95–105%, and a relative standard deviation of 1.8%–5.6% [10].
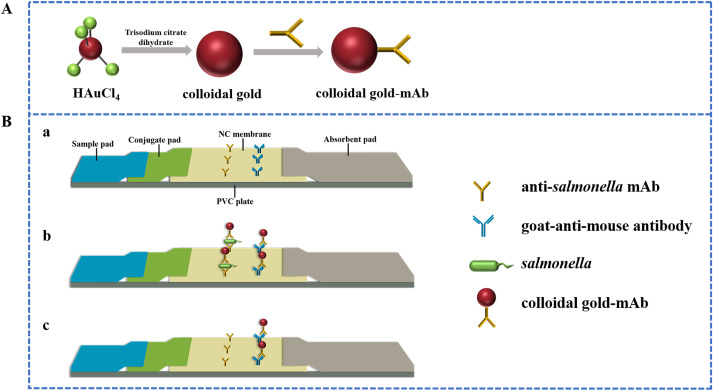
Figure 3. Preparation and detection principle of Salmonella immunochromatographic test strip [10].
4. Advantages and disadvantages
4.1. Advantages of colloidal gold
Colloidal gold has several advantages, such as high sensitivity, quick response, specific recognition, easy operation and visual detection [11]. Due to their potent surface plasmon resonance effect, colloidal gold nanoparticles have distinctive optical characteristics that give them their vibrant hue. With the ability to see this color change with the naked eye, target analytes can be detected quickly and accurately [12]. Colloidal gold-based assays offer excellent sensitivity and specificity. The large surface area of gold nanoparticles provides ample binding sites for the immobilization of capture molecules, resulting in enhanced signal amplification [13]. Moreover, the unique physicochemical properties of gold nanoparticles, such as their stability and biocompatibility, ensure low background noise and minimize nonspecific interactions, leading to high assay specificity. Colloidal gold also has various biomedical applications, such as wound healing, drug delivery, tumor detection, gene therapy, photothermal therapy and biosensing.
4.2. Disadvantages of colloidal gold
Colloidal gold also has some limitations, such as possible aggregation, clearance and immune response in vivo, which need to be further addressed by optimizing the synthesis and functionalization methods. Depending on the size, shape, and surface chemistry of the nanoparticles in colloidal gold, there may be some toxicity and risks associated with its production and use. Colloidal gold may also interact with other drugs or supplements in the body, causing adverse effects or reducing their efficacy.
5. Conclusion
Colloidal gold is a versatile nanomaterial that has been widely used in various biomedical fields, especially in cancer treatment and diagnosis. It can be synthesized in different sizes and shapes, and can be modified with various functional molecules to achieve specific targeting, imaging, drug delivery, photothermal therapy and biosensing. Colloidal gold has many advantages over other nanomaterials, such as good biocompatibility, low toxicity, high stability, easy preparation and surface modification. However, analytical techniques based on colloidal gold also have some limitations, such as possible aggregation, clearance and immune response in vivo, which need to be further addressed by optimizing the synthesis and functionalization methods. Therefore, colloidal gold is a promising nanomaterial for biomedical applications, but also requires more research and development to overcome its challenges and improve its performance.