1. Introduction
High-fat diet (HFD) is one of the main environmental drivers of obesity, insulin resistance, non-alcoholic fatty liver disease (NAFLD) and cardiovascular disease in modern society. Long-term HFD intake leads to excess energy, causing adipose tissue dysfunction, systemic chronic low-grade inflammation and metabolic disorders in multiple organs [1, 2]. The liver, as a core organ for energy metabolism and lipid processing, is a key target of HFD damage, manifested as hepatic steatosis (fatty liver), inflammatory infiltration, and even progression to steatohepatitis and fibrosis [3, 4].
Increasing evidence shows that the immune system, especially the adaptive immune response, plays a central role in HFD-induced metabolic inflammation and organ damage [5, 6]. HFD can change the composition and activity of immune cells in metabolic organs such as adipose tissue and liver, such as promoting the differentiation of pro-inflammatory Th17 cells, inhibiting the function of regulatory T cells (Treg), and exacerbating local and systemic inflammation [6]. Winer et al. [5]'s groundbreaking study showed that immunotherapy targeting T cells can significantly improve obesity-related insulin resistance, suggesting that adaptive immunity is a key mediator of metabolic pathology. Recombinase activating gene 1 (Rag1) knockout (Rag1-/-) mice completely lack mature T lymphocytes and B lymphocytes due to V(D)J recombination defects, and are an important model for studying adaptive immune function. Liu et al. [7] found that the administration of exogenous adiponectin can prevent weight gain and abnormal glucose metabolism in Rag1-/- mice fed with HFD, suggesting that the model itself may have a certain resistance to HFD-induced metabolic disorders, but its effect on the liver, especially the expression of the key lipid transport protein apolipoprotein E (ApoE) in the liver, is still unclear.
ApoE is crucial in lipid metabolism, especially reverse cholesterol transport and lipoprotein remnant clearance. ApoE-/- mice are a classic model for studying atherosclerosis, and HFD can further aggravate its vascular lesions [4, 8] and retinal damage [9]. Studies have shown that HFD itself can affect ApoE expression or function in multiple tissues (such as adipose tissue, aorta, and retina) [4, 8, 10], but in the liver, the main synthetic organ, the regulatory effects of HFD and adaptive immune status on ApoE expression still need to be further explored.
Based on this, this study used the Rag1-/- mouse model, combined with 8 weeks of HFD intervention, to clarify: 1) the effect of adaptive immune deficiency on HFD-induced systemic obesity (weight gain) and liver weight/morphological changes; 2) the regulatory effect of HFD and adaptive immune deficiency on liver ApoE gene expression. This study will help to deepen the understanding of the mechanism of action of adaptive immunity in HFD-related metabolic liver disease and provide experimental basis for finding new intervention targets.
2. Materials and methods
2.1. Mice and high-fat diet
Male C57BL/6J mice and Rag1-/- mice (6 weeks old) were housed, bred, and maintained under specific pathogen free conditions, were randomly assigned to either a high-fat diet (HFD; 45% kcal from fat, cat, 160302, Sibeifu Beijing Biotechnology Co., Ltd.) or normal diet (ND; 4-10% kcal from fat) for 8 weeks. Mice were housed in a controlled environment (12-hour light/dark cycle, 22 ± 1 °C) with food and water provided throughout the study period. Body weight were monitored at day 0, day 18, day 32, day 47, and mice were sacrificed and dissected at day 55. All experiments complied with the relevant laws and institutional guidelines.
2.2. RNA isolation, real-time–qpcr analysis
To quantify mRNA expression, total RNA was extracted from liver tissues using the Trizol total RNA isolation reagent (Invitrogen), and specific quantitative real-time PCR experiments were performed using the TransScript II Green One-Step qRT-PCR SuperMix (Transgen), according to the manufacturer’s instructions. 18S ribosomal RNA (18S-F, GTAACCCGTTGAACCCCATT, 18S-R, CCATCCAATCGGTAGTAGCG) expression levels were determined as internal controls. Fold change in expression level of ApoE gene (ApoE-F, CTCCCAAGTCACACAAGAA, ApoE-R, TGTTCCTCCAGCTCCTTT) was calculated using the 2−∆∆Ct method.
3. Results
3.1. Changes in body weight, liver weight and morphology of mice
After 8 weeks of feeding, the weight changes of mice in each group showed an upward trend over time (Tables 1, Figures 1). Compared with the normal-diet (ND) group, the weight of mice in the HFD group did not increase significantly, which was inconsistent with the typical characteristics of HFD-induced obesity. Regardless of the ND or HFD group, the basal weight of Rag1-/- mice increased more slowly over time compared with the WT group. This result is consistent with the partial resistance to HFD-induced weight gain observed by Liu et al. [7] in Rag1-/- mice, suggesting that the loss of mature T and B lymphocytes weakens the effect of HFD on promoting obesity. The results are shown in Tables 1, Figures 1.
HFD significantly increased the absolute liver weight of Wt mice (Table 1-2, Figure 2, Wt-ND vs Wt-HFD, p =0.0176). Morphologically, HFD caused the liver to be pale and lack blood color (Figure 3), which is a typical manifestation of liver fat accumulation (fatty liver). In Rag1-/- mice, HFD also significantly increased the absolute liver weight (Table 1, Figure 2, Rag1-/--ND vs Rag1-/--HFD, p =0.0405). Similarly, HFD caused the liver to be pale and lack blood color (Figure 3). However, the liver weight in Rag1-/- mice was lower than that in the Wt group, regardless of the ND group or the HFD group. This suggests that Rag1 deficiency may affect liver development.
Table 1. Mice body and liver tissue weight
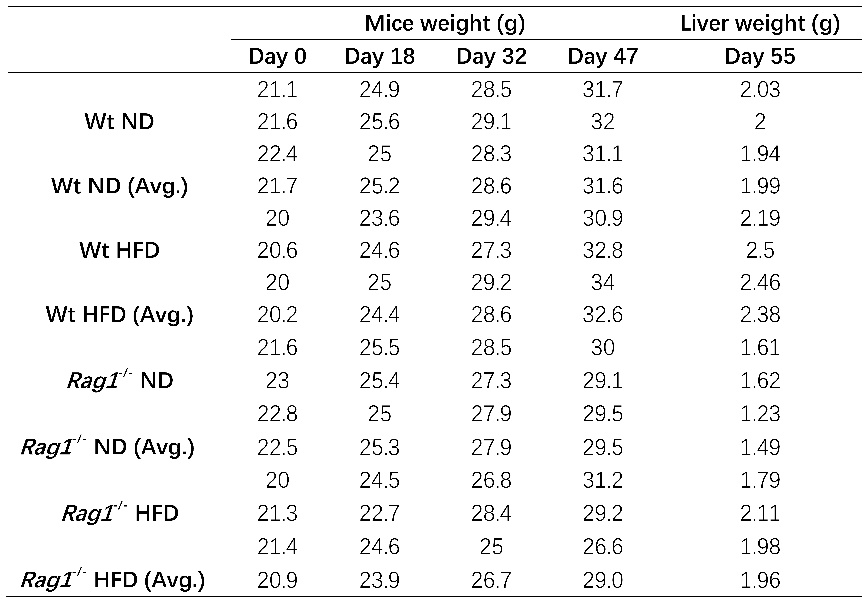
Note: Table1 listed mice body (4 different time points since HFD starts at day 0) as well as liver tissue weight (n=3) at day55 (sacrificed time point), average values from 3 mice was also calculated and listed.
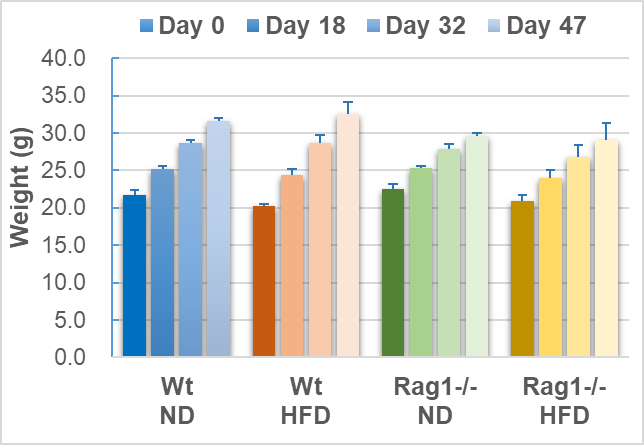
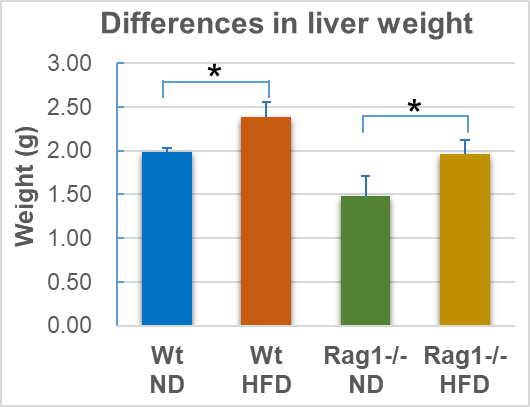
Note: Average of body weight or liver weight (n=3), as well as standard deviation (stdev.s) was calculated as mentioned in methods, * means p<0.05 (t.test).
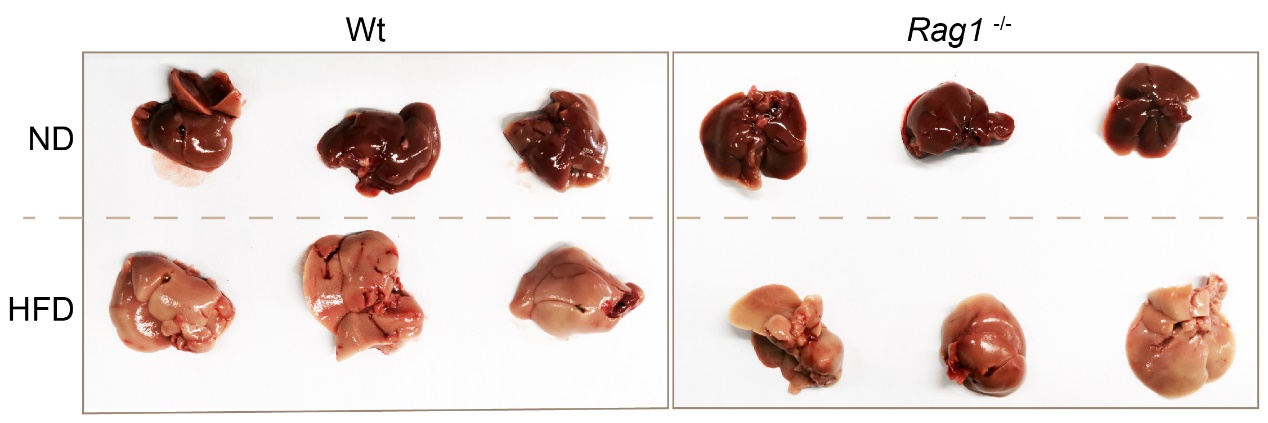
Note: top row was ND liver, bottom row was HFD liver, left panel was Wt mice (n=3), right panel was Rag1-/-mice (n=3), liver tissue was removed from mice fed with HFD for 8 weeks.
3.2. Apoe mrna expression level in liver
qRT-PCR was used to detect the expression of ApoE gene in liver (Tables 3-4, Figure 4). HFD feeding resulted in a near 50% off but insignificant downregulation of ApoE mRNA expression levels in the liver of the Wt-HFD group mice (fold gene expression changes from 1.05 to 0.5, p =0.077 compared with the Wt-ND group). The basal ApoE expression levels of the Rag1-/--ND group (0.57) were similar to the Wt-HFD (0.50) group, indicating that Rag1 knockout leads to decreased ApoE expression. Similarly, in the context of Rag1 deficiency, HFD feeding resulted in further declination of ApoE expression (from 0.57 to 0.35, Table 4). This result strongly suggests that HFD leads to repression of ApoE expression, and the presence of mature lymphocytes (T, B cells) or the inflammatory response mediated by them also correlates with the expression of ApoE. The data are shown in the Tables 2 and Figure 4.
Table 2. Ct values and calculates of fold gene expression
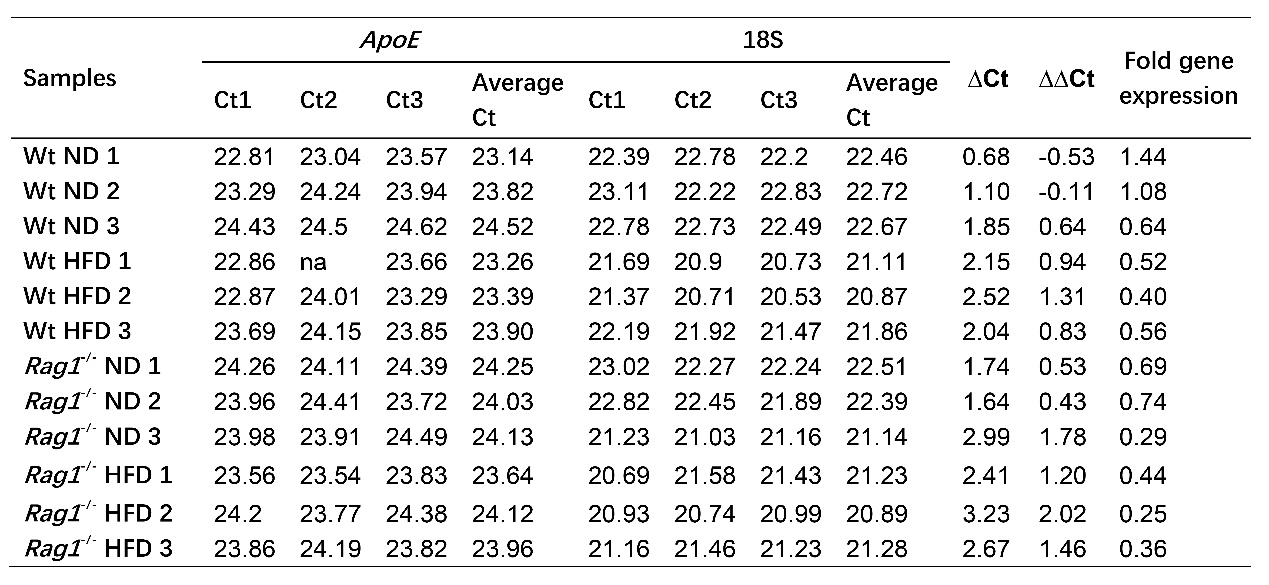
Note: 18S was chose as internal control, and the average of Wt ND ∆Ct was used for control to deduce ∆∆Ct.
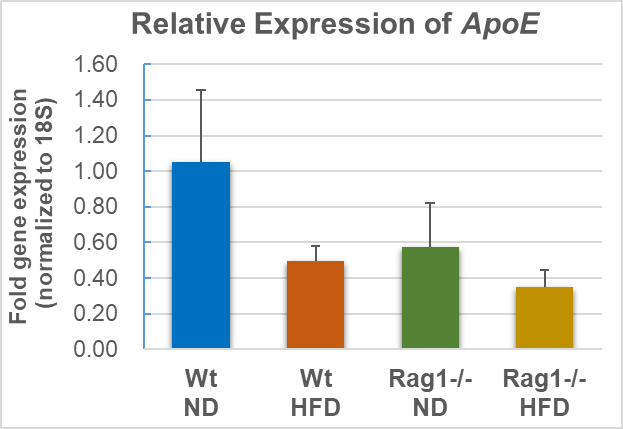
Note: Average of fold gene expression (n=3), as well as standard deviation (stdev.s) was calculated as mentioned in methods, t.test was applied for calculation of p between Wt-ND vs Wt-HFD (p=0.077), as well as Rag1-/--ND vs Rag1-/--HFD (p=0.211).
4. Conclusion and perspective
4.1. Conclusion
This study compared the metabolic phenotypes, especially liver changes, of wild-type (Wt) and Rag1 knockout (Rag1-/-) mice after 8 weeks of HFD intervention, revealing the key role of the adaptive immune system in HFD-induced metabolic disorders and liver damage, and for the first time linking the downregulation of liver ApoE expression with this process and the adaptive immune state.
We observed that HFD can significantly downregulate liver weight in wild-type and Rag1-/- mice, and the loss of mature T and B lymphocytes (Rag1-/-) is potentially associated with HFD-induced excessive weight gain and relative liver enlargement, as well as changes in ApoE mRNA expression levels.
In summary, this study suggests that the adaptive immune system is a potential driving factor for HFD-induced systemic obesity and the suppression of key lipid transporter ApoE expression.
4.2. Perspective
From the perspective of research significance, it is worth noting that genetic background (such as ApoE genotype) significantly affects the effect of HFD. Jones et al. [11] found that HFD only increased gliosis and immediate early gene expression in the brain of ApoE3 mice, but not in ApoE4 mice, highlighting the regulatory role of ApoE genotype in neuroinflammation. Although this study did not change the ApoE gene itself, it revealed that in the context of physiological ApoE expression, HFD regulates its expression level through adaptive immune-dependent pathways, further enriching the complex role of ApoE in metabolic-immune interactions.
In addition, the effects of HFD on the body are significantly tissue-specific. This study found that liver ApoE expression is regulated by HFD and immunity, while Zou et al. [10] reported that HFD significantly changed retinal lipid composition and gene expression networks, and Cao et al. [9] found that HFD combined with ApoE deficiency damaged the retina. Studies by Keleher et al. [12] and Alradi et al. [2] respectively highlighted the profound effects of HFD on the epigenome (DNA methylation) and spatially specific gene expression in adipose tissue. Together, these studies depict a complex spectrum of effects of HFD at multiple organs and levels (gene expression, epigenetics, lipidomics).
Looking forward to the future, there are many directions worthy of further in-depth research. Targeting the adaptive immune response, especially regulating specific T cell subsets or related inflammatory pathways, may become a new strategy for preventing and treating HFD-related metabolic liver diseases (such as NAFLD/NASH) and their cardiovascular complications. Future studies need to further analyze the specific lymphocyte subsets that mediate HFD inhibition of liver ApoE expression and the key effectors of their secretion, and explore the long-term effects of intervening these targets on metabolic health.



