1. Introduction
About 20% to 25% of the world's electricity is used for lighting, and LED is concerned because of its energy-saving advantages. The traditional inorganic LED preparation conditions are strict and high cost, while the efficiency of OLED decreases at high current. Therefore, finding highly efficient and low-cost photoelectric materials has become a research hotspot.
Perovskite is applied in several fields because of its photoelectric properties. Nano-sized perovskite quantum dots exhibit better luminous characteristics, and their electronic band gap structure is affected by the quantum domain effect. Quasi-zero-dimensional perovskite quantum dots can regulate optical and electrical properties and improve the probability of exciton formation. The LED performance of perovskite quantum dots depends on the quality of the light emitting layer, which can be improved by optimizing the preparation method and adjusting the composition. Quantum dot thin film has a high color gamut, pure color and stable performance, so the photovoltaic devices based on this have better performance.
This paper summarizes all-inorganic perovskite quantum dot materials, organic and inorganic perovskite and applications, discusses LED principles, preparation methods and EQE strategies for improving LED, including surface ligand engineering, interface modification, passivation and energy level alignment, and summarizes the prospect of perovskite quantum dots LED in display and lighting, as well as stability and toxicity challenges.
2. All-inorganic perovskite quantum dots
All-inorganic perovskite quantum dots (IPQDs) have higher stability than organic-inorganic hybrid perovskite quantum dots. IPQDs have high light absorption coefficient, narrow emission spectrum, high photoluminescence quantum yield (PLQY), adjustable composition and size, and adjustable emission spectrum, with excellent photoluminescence and electroluminescence characteristics, so it has attracted much attention. As one of the most promising optoelectronic materials, IPQDs are widely used in LEDs, solar cells, photodetectors, lasers and other fields.
2.1. The morphology, optical properties and surface properties of the nanocrystalline crystals
The morphology and size of nanocrystalline shape their properties. The main ways to regulate the morphology of IPQDs are: changing the surface ligand (type and dosage) and controlling the reaction time. The surface chemistry of CsPbBr3 and found that amine ligands were easy to fall off during polar solvent washing, and the more soluble cesium acetate as a precursor could improve the preparation performance. Table 1 shows the morphology and size of CsPbBr3 prepared sample at 140℃and 170℃. By controlling the reaction time and obtain CsPbBr3 with different morphology at 150℃, surface ligands and reaction time are the key to regulate the morphology and size of IPQDs to meet different application requirements.
Table 1. Summary of the morphology and size of CsPbBr3 nanocrystals obtained from different acid-base combinations [1]
Samples | Temperature/℃ | Morphology | Size/nm |
C18A-C18B | 170 | Nanocube | 7.1±0.8a |
C18A-C18B | 140 | Nanoplatelet | 2.6±0.3b |
C12A-C18B | 170 | Nanocube | 9.5±1.2a |
C8A-C18B | 170 | Nanocube | 12±1.8a |
C6A-C18B | 170 | Nanocube | 13±2.3a |
C6A-C18B | 140 | Nanoplatelet | 2.6±0.4b |
C2A-C18B | 120 | Nanoplatelet | / |
C18A-C12B | 170 | Nanoplatelet | 4.5±0.6b |
C18A-C8B | 170 | Nanoplatelet | 3.5±0.4b |
C18A-C8B | 140 | Nanoplatelet | 2.6±0.3b |
C18A-C6B | 140 | Nanoplatelet | 1.8±0.3b |
Note: a—Average edge length of nanocube, b—Average thickness of nanoplatelet | |||
At present, the main ways to regulate the emission wavelength of IPQDs are:
(1) Change the halogen composition: With the change of ClBrI, its emission spectrum redshifts and widens.
(2) Using quantum domain effect: regulating the luminous wavelength by regulating the size of quantum dots.
Specifically, the CsPbCl3, CsPbBr3 and CsPbI3 nanocrystals emit blue, green and red light, respectively. By changing the PbX2 scale or mixing different halogen quantum dot solutions can synthesize mixed halogen perovskite nanocrytes. By changing the reaction temperature, the quantum dot size increases with temperature and the luminous wavelength also redshifted with size. This is one of the unique advantages of the IPQDs.
2.2. CsPb X3 problems with nanocrystalline crystals
Ligands on the surface of QDs are easily shed in polar solvents, leading to the decomposition or agglomeration of iotropic IPQDs, and fluorescence quenching. Therefore, CsPbX3 saves only in nonpolar solvents such as hexane, limiting its application to [2]. The poor light resistance of IPQDs may be the light-promoted ionization, oxidation, and agglomeration of [3]. Huang et al. studied the effects of light, oxygen, humidity and temperature on the degradation of CsPbBr3 nanocrystalline, and found that green nanocrystalline quickly turned yellow and weakened under light. After isolating the oxygen, the color change slows down. Under air conditions, increased light and humidity can accelerate nanocrystal degradation. In conclusion, light, oxygen, and humidity together accelerate the degradation of IPQDs in purification, storage, and application (Table 2).
Table 2. Summary of the stability based on inorganic halide perovskite-based materials [4-13]
Materials | Methods | Stability |
CsPbBr₃ | Silica/Alumina monolith | Light |
CsPbX₃/ZnS | Heterostructure | Chemical stability |
CsPbBr₃ | Polymeric matrices | Water and light |
CsPbBr₃ | POSS | Water |
CsPbBr₃ | Mesoporous silica | Chemical stability |
CsPb(Br or I)₃ | APTES | Air |
CsPbX₃ | Polymer matrix | Water |
CsPbBr₃ | Alkyl phosphate | Water and heat |
CsPbX₃: Mn²+ | Doping of Mn²+ | Thermal stability |
CsPbX₃ | Polymer | Chemical stability |
The existence of lead in IPQDs has always been an unavoidable problem. At present, researchers are conducting research: the appropriate non-toxic or low toxic cations partially or completely replace lead, hoping to fundamentally solve the problem, such as CsSnX3 with suitable band gap was considered as a potential surrogate for the [14].
2.3. Application
For their great potential in lighting and display, with dual properties of photoluminescence and electroluminescence, they can be used to manufacture LEDs. When preparing white LEDs, IPQDs with different halogen compositions should be used to avoid anion exchange. White light LEDs were prepared by dispersing green and red IPQDs into a polymethylmethacrylate chloroform solution, applying them to a blue light chip and drying them under vacuum.
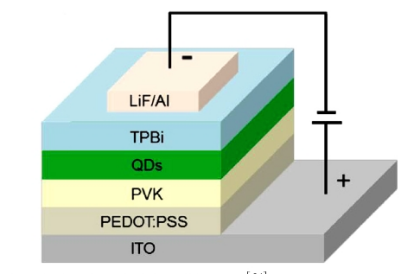
Figure 1. Illustration of multilayer perovskite QLED device [15]
Perovskite solar cells were prepared from lead-halogen perovskite nanocrytes (Figure 1). Its low carrier recombination rate, high mobility, long diffusion distance, and life makes it suitable for efficient solar cells. The photoelectric conversion efficiency of organic lead halovskite materials originates from excellent properties such as bandgap, light absorption, defect tolerance, carrier diffusion distance, low exciton binding energy, and balance of electron and hole mobility.
However, researchers are also thinking about the high-efficiency perovskite solar cells, CH3NH3+ is it required or not. In addition, Beal et al. [16] reported the use of CsPb in solar cells structured as shown in Figure 2. As a light-absorbing layer, a photoelectric conversion efficiency of 6.5% was achieved. There is still a gap between the efficiency of fully inorganic perovskite solar cells and organic lead halogen perovskite solar cells. Currently, in most methods in the literature concerning the optical absorption layer in perovskite solar cells, heat treatment is essential.
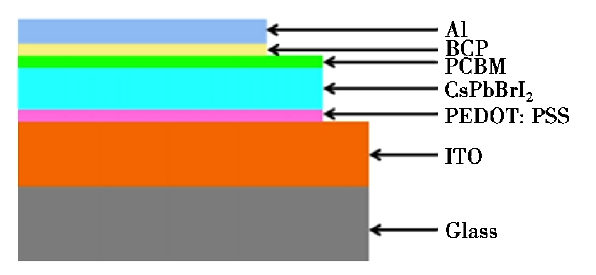
Figure 2. Device structure of perovskite solar cell [16]
Song et al. [17] used ultra-thin two-dimensional CsPbBr3 nanosheets to prepare high-performance flexible photodetectors. The device has excellent electron migration performance, with a high light on/off ratio (>103) and strong flexibility (>10,000 cycles). Working with a 442 nm wavelength laser for 12 hours, photosensitivity fluctuated by 2.6%. The light trapping and carrier flow migration capabilities of the photosensitive layer are crucial for optoelectronic devices.
The photodetector made with this film (Figure 3) has an external quantum efficiency of 658% at 520 nm wavelength and 9V bias, significantly improving its performance compared to the perovskite photodetector reported previously [18]. The above studies fully demonstrate the great potential of IPQDs in the field of photoelectric detection.
IPQDs (inorganic perovskite quantum dots) have attracted much attention in the field of laser applications because of their high fluorescence quantum yield, low threshold at room temperature, wavelength tunability and ultra-stable excited emission characteristics. Figure 4 illustrates in detail the optical system [19] of the laser setup. As an emerging semiconductor material, IPQDs provide a strong material basis for the research and development of laser devices with their stable structure and adjustable photoelectric performance.
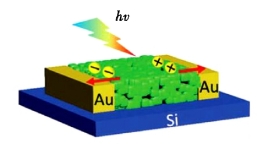
Figure 3. Photodetectors based on the Mie porous and interface-fused perovskite films [20]
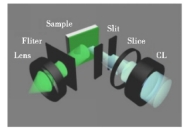
Figure 4. Schematic of the modified optical systems for pumping configuration, cylindrical lens(CL) [21]
3. Metal-organohalides CH3NH3PbX3 (X = Cl, Br, I) quantum dots
Among the wide variety of quantum dots, quantum dots based on perovskite structure have attracted great attention for their excellent luminescence performance, such as high luminescence efficiency and narrow generation spectral line. Organic-inorganic hybrid of the CH3NH3PbX3 (X = Cl, Br, I) is also the key perovskite quantum dot.
3.1. Metal-organohalide CH3NH3PbX3 (X = Cl, Br, I) Structure and optical properties of the quantum dots
Taking CH3NH3PbBr3 as an example, this paper introduces the crystal structure of the quantum dots of CH3NH3PbX3(X=Cl, Br, I). Symsymmetric regular octahedral structure, Pb in the center of the octahedron, CH3NH3+ distributed in the center of the octahedral network connected by the top angle, as shown in Figure 5. At room temperature CH3NH3PbI3 is generally cubic crystal systems, while at room temperature, CH3NH3PbI3 is generally tetragonal, and changes to cubic crystal [22] at 55~57℃.
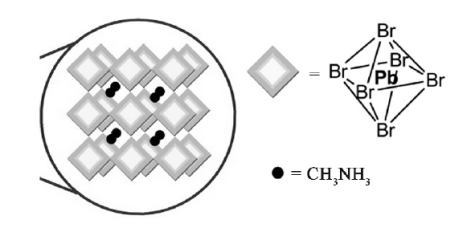
Figure 5. Schematics of an array of corner sharing MX6octahe-dra confined in the three dimensions due to the or ganic capping [22]
After passivation by oleic acid and octylamine, the quantum dots have modifier molecules on the surface and are connected to PbBr6 around the octahedron (Figure 6). Unable to form CH3NH3PbBr3 quantum dots without n-octyl amine; without refueling acid, the solution is cloudy and unstable. Octylamine modifiers control the crystallization dynamics and the quantum dot size; oleic acid prevents the quantum dot aggregation and ensures the stability of the colloidal system. A similar conclusion by Gonzalez-Carrero et al. [23] despite the similar synthesis methods, the QDs vary in size.
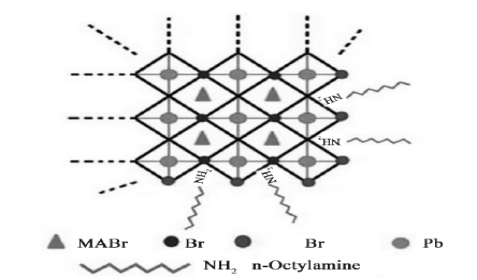
Figure 6. Molecular structural model [24]
CH3NH3PbBr3 quantum dots have very high fluorescence quantum efficiency, with the reported values in different literature exceeding 70% and the highest exceeding 90%. This is in conjunction with the band structure of CH3NH3PbBr3 itself, CH3NH3PbBr3 is a direct bandgap semiconductor, the exciton radiation recombination probability itself is high. Further analysis by F. Zhang et al [24]. On the one hand, they tested the quantum dot variable temperature photoluminescence, and found that the exciton binding energy of the quantum dot is about 375 meV, much more than the 65 meV of the bulk material, and it will increase as the size of the quantum dot decreases. In summary, the enhanced exciton binding energy and the favorable blunt effect surface work together to enable CH3NH3PbBr3. The fluorescence quantum efficiency of quantum dots exceeds 70% (Table 3).
Table 3. Optical properties of CH3NH3PbBr3 quantum dots in different references
Excitation wave | Hair light wave | Light peak half | Fluorescence quantum |
long /nm | long /nm | High width /nm | productiveness /% |
450 | 515 | 21 | 70 |
365 | 475~520 | 28~36 | 74~93 |
365 | 527 | 21 | 17 |
350 | 513 | 32 | 83 |
3.2. Application
Perovskite materials have high photoluminescence efficiency, so researchers have begun to apply perovskite materials to LED in the past two years. In August, 2014, the application of perovskite materials to LED devices was reported by Z.K.Tan et al. [25]. They prepared LED devices in the infrared and visible bands. In June 2015, N.K. Kuwamat et al [26] were the first to report blue light LED devices based on perovskite materials. They use CH3NH3PbClxBr3-x as EML, the LED device device emits 482 nm blue light, but the ηEQE of the device is not high. In December, 2015, the H. Cho et al. [27] reported a ηEQE of up to 8.53% of LED green light devices (Table 4).
Table 4. Latest advances in LED device with a perovskite struc-ture based on CH3NH3PbX3 (X=Cl, Br)
EML | λ/nm | EQe¹% |
MAPbBr₃ | 542 | 0.76 |
MAPbCl I₃- | 754 | 0.1 |
MAPbBr₃ | 525 | 0.125 |
MAPbI₃ | About 770 | 0.04 |
MAPbBr₃ | 525 | |
MAPbCl₀.44Br₁.37I ₁.19 | 760 | 0.28 |
MAPbBr₃ | 532 | 0.8 |
MAPbCl I₃- | 768 | 3.5 |
MAPbCl I₃- | 775 | 0.024 |
MAPbBr₃ | 534 | 1.2 |
MAPbBr₃ | 536 | 0.1 |
MAPbCl I₃- | About 775 | 0.48 |
MAPbBr₃ | 530 | 0.051 |
MAPbCl Br₃-x | 482 (Blue) | 0.0003 |
MAPbBr₃/PEO | 535 | 0.083 |
MAPbI₃ | 775 | About 0.9 |
MAPbBr₃ | About 540 | 8.53 |
4. Conclusion
Perovskite quantum dots, because of their excellent optical performance, have become the ideal photoelectric materials in many display and lighting equipment. Based on perovskite quantum dot LED EQE. The growth has been to 20% in four years, and the service life has been extended to tens of hours, making perovskite quantum dots LED in solid state lighting and display a very promising device. This paper summarizes the recent research progress based on different types of perovskite quantum dots in LED, introduces the properties, existing problems and applications of all inorganic perovskite quantum dots; discusses organic and inorganic perovskite and applications, and discusses some methods to improve the LED efficiency of perovskite quantum dots. Finally, the current challenges of the LED of perovskite quantum dots are analyzed. In this paper, it is found that the luminescence efficiency of perovskite LED is no longer an obstacle to display application, but perovskite quantum dot LED still faces several challenges. (1) The EQE of green light, red light and near-infrared optical perovskite quantum dot LED is over 20%, but the development of blue light LED is facing challenges. The blue luminous perovskite is achieved by Cl/Br mixing, with adjustable emission color. However, the mixed Cl/Br perovskite easily induced phase segregation, which reduces PLQE and affects LED efficiency and color stability. (2) The poor stability of perovskite materials has seriously limited their large-scale use. Perovskite quantum dots are prone to temperature, humidity, light and other phase change, agglomeration and even degradation. (3) In the widely studied and outstanding perovskite, the lead ion is a heavy metal ion that is harmful to the body, and its toxicity restricts the practical application of perovskite LED. In order to reduce or remove toxicity, Sn, Mn, Bi and Sb can be used as alternative elements for Pb, such as isovalent Sb substitution and crystal evolution, new inorganic Sb-based perovskite single crystals. In the future, it will be a new research trend to improve the color and environmental stability of perovskite LED and to develop environmentally friendly and efficient perovskite LED.



