1. Introduction
Endless and inexhaustible, solar energy is considered one of the most promising clean alternatives to traditional fossil fuels [1]. Among the developed solar energy technologies, photovoltaic technology can directly convert solar energy into electrical power [2]. Meanwhile, photo(electro)catalysis can transform solar energy into combustible fuels such as hydrogen, carbon monoxide, ammonia, etc. [3]. However, these technologies need to be connected to external devices (like rechargeable batteries and supercapacitors) to compensate for the intermittent availability of sunlight, ensuring continuous operation. This integration results in bulky systems that are cumbersome [4], and mismatches in current and voltage between devices, as well as energy losses due to external wiring, reduce the overall energy efficiency of the system [5]. Improving energy utilization efficiency by integrating solar conversion and storage within the same device is becoming increasingly important.
Now, using photoactive materials designed as single-device dual-function photoactive electrodes, solar energy can be converted and stored directly within the electrodes. These dual-function materials typically operate in two steps: (1) absorb sunlight and generate electron-hole pairs; (2) the light-generated holes/electrons further transfer energy to electrochemical energy storage materials during the charging and discharging process [6]. Therefore, the capabilities of photoactive electrode materials, such as light harvesting, energy level matching, and reversible electrochemical reactions, determine the cost, stability, and solar energy output storage efficiency of energy storage systems.
Copper catecholate (Cu-CAT), a copper-based metal-organic framework (MOF) material, possesses numerous advantages such as abundant metal active oxidation sites, large specific surface area, and permanent porosity, and also features strong broadband light absorption [7], exhibiting both photo-sensitivity and pseudocapacitance properties. Given its high metallic conductivity among MOF materials [8], this paper initially verifies the potential of Cu-CAT as a photoactive electrode material. Utilizing its characteristics as a supercapacitor, it is prepared as a photoactive cathode [9]. The zinc foil, made into an anode with battery-like characteristics, is combined with the cathode to form an aqueous zinc-ion hybrid supercapacitor that boasts both the high capacity density of batteries and the high power density of supercapacitors [10,11]. Under simulated sunlight conditions, this enhances the energy density of the hybrid supercapacitor.
2. Experiment
2.1. Reagents and Equipment
Reagents:
Copper (II) acetate monohydrate (C4H6CuO4·H2O), analytical grade, Shanghai Aladdin Reagent Company; Hexahydroxytriphenyl (HHTP), analytical grade, Shanghai Aladdin Reagent Company; Acetone (C3H6O), analytical grade, National Medicines Group Chemical Reagent Company; N,N-Dimethylformamide (DMF), analytical grade, National Medicines Group Chemical Reagent Company; Anhydrous ethanol (C2H6O), analytical grade, National Medicines Group Chemical Reagent Company; Zinc sulfate heptahydrate (ZnSO4·7H2O), analytical grade, Shanghai Aladdin Reagent Company; Potassium chloride (KCl), analytical grade, Shanghai Aladdin Reagent Company; Deionized water (H2O), analytical grade, Suzhou Nano Research Institute; Hydrophilic carbon cloth, 0.31 mm, Suzhou Su Ke Precision Instrument Company.
Equipment: Cold field scanning electron microscope (SEM), Hitachi S-4800, Japan Hitachi Instruments; Ultraviolet photoelectron spectroscopy (UPS), ESCALAB 250XI, Thermo Fisher Scientific, USA; Xenon lamp light source system, CEL-PF300-T8, Beijing Zhong Jiao Jin Yuan Technology Company; Electrochemical workstation, CHI 660E, Shanghai Chenhua Instruments.
2.2. Synthesis Method
Carbon Cloth Treatment: Cut hydrophilic carbon cloth (abbreviated as CC) into 1 cm × 2 cm pieces, sequentially ultrasonicate in acetone, anhydrous ethanol, and deionized water for 5 minutes each. Set up a three-electrode system with the carbon cloth as the working electrode and dilute sulfuric acid as the electrolyte. Perform hydrophilization treatment using the CHI660e electrochemical workstation, then soak the treated carbon cloth in deionized water until needed.
Precursor Solution Preparation: Weigh out 0.06 mmol (12 mg) of copper (II) acetate monohydrate and 0.03 mmol (9.75 mg) of hexahydroxytriphenyl (HHTP) powders. Dissolve separately in 0.5 mL deionized water and 0.5 mL N, N-dimethylformamide (DMF), ultrasonicate and stir vigorously for 30 minutes.
Cu-CAT@CC Electrode Fabrication: Place the treated carbon cloth into a 50 mL high-pressure autoclave, add the precursor solution, and conduct hydrothermal treatment in an oven at 85°C for 8 hours. After the hydrothermal reaction, cool and retrieve the carbon cloth, rinse three times with deionized water, and dry in an oven at 60°C for 6 hours to obtain the final Cu-CAT@CC electrode.
3. Results and Discussion
3.1. Structural Characterization
The morphology of Cu-CAT@CC was observed under a scanning electron microscope (SEM), as shown in Figure 1, with Figures 1a and 1b displaying magnifications of 50k and 20k, respectively. From Figure 1a, it is evident that the Cu-CAT forms a nanorod array on the surface of the carbon cloth fibers, with diameters in the tens of nanometers. During the hydrothermal process, the hydrophilized carbon fiber surface binds numerous oxygen-containing functional groups, thereby filling with active sites. Precursor solution forms nucleation sites at these active spots and then continues to grow along the original orientation. The duration of hydrothermal treatment determines the length of the nanorods; overly prolonged growth times cause the material to agglomerate, losing its regular morphological features. Additionally, Figures 1a and 1b show large clusters of nanorods gathering together, forming regular nano-flower structures, suggesting that during the hydrophilization process, active sites densely accumulate, and multiple nuclei grow concentrated at these nearby active sites.
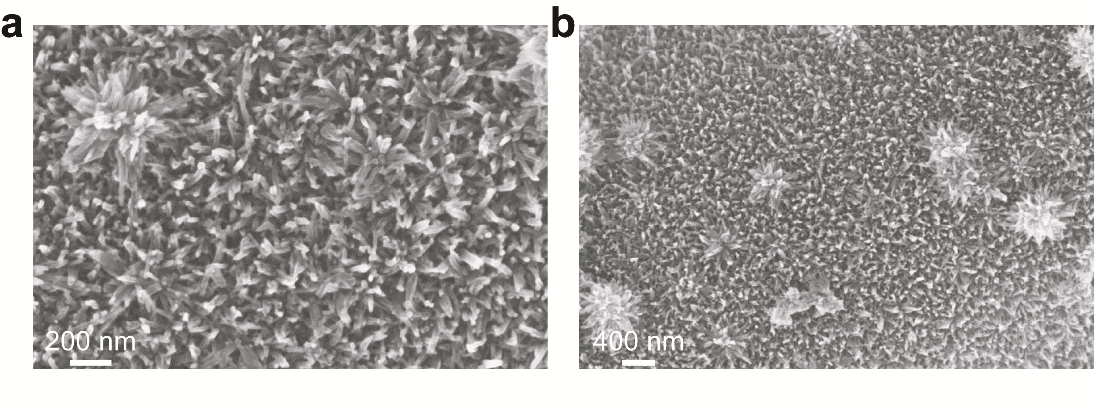
Figure 1. SEM images of Cu-CAT nanorod arrays at 50k (a) and 20k (b) magnification
Photoactive electrode materials generally should possess the following characteristics: (1) an appropriate band gap (1.8-3.0 eV) to absorb more visible light, (2) good transparency, (3) high efficiency in photo-generated electron-hole separation, and (4) long-lived photo-generated holes [6]. To validate that Cu-CAT MOF material meets the characteristics of photoactive electrode materials, the UV-visible light absorption spectrum (UV-vis) of Cu-CAT@CC was tested. The results, as shown in Figure 2a, indicate that the material has its strongest absorption peak around the 365 nm band. According to the semiconductor structure band theory, the band gap Eg is calculated to be 2.54 eV (Figure 2b), aligning with the fundamental characteristics of a photoactive electrode material's band gap.
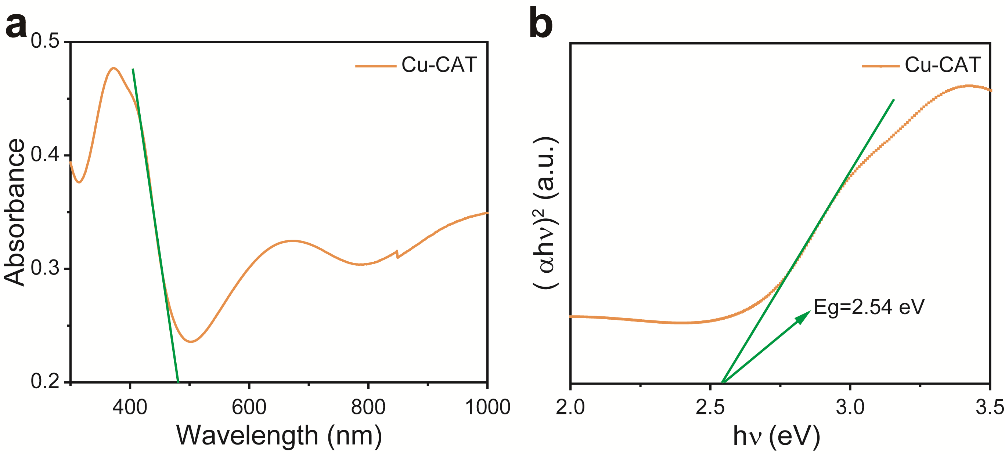
Figure 2. a. UV-vis spectrum of Cu-CAT; b. Eg value
After determining the Eg of Cu-CAT to be 2.54 eV, it is necessary as a semiconductor material to establish the relative positions of its conduction band (CB) and valence band (VB). By testing the ultraviolet photoelectron spectroscopy (UPS) of Cu-CAT@CC, the approximate ranges for CB and VB were estimated. As calculated and shown in Figure 3, the work function is determined to be 5.11 eV, and the VB edge is also precisely 2.54 eV from the Fermi level (EF). By setting the vacuum energy level (EVAC) to zero, the final determination places the CB at -5.11 eV and the VB at -7.65 eV.
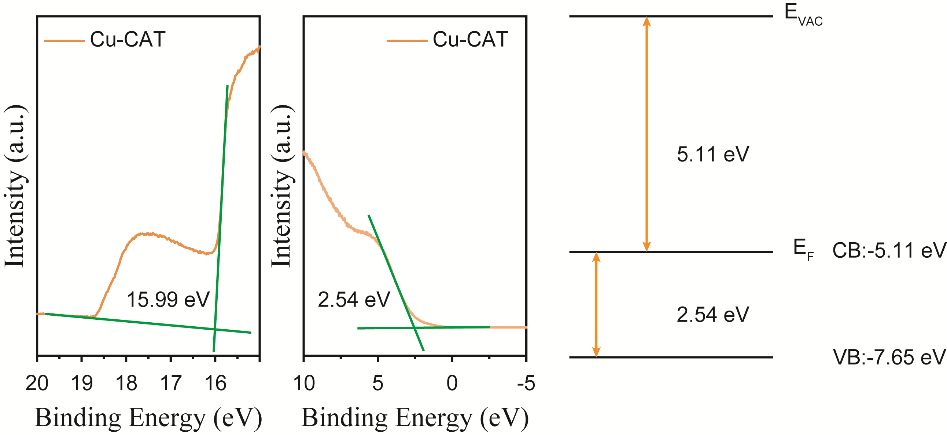
Figure 3. UPS spectra of Cu-CAT with CB and VB values
3.2. Electrochemical Performance
To assess the electrochemical performance of Cu-CAT@CC as a photoactive electrode, a three-electrode test system was set up as shown in Figure 4. The testing was conducted using a chi660e electrochemical workstation in an electrolytic cell with 85% transparency. The working electrode was a 1 cm × 2 cm Cu-CAT@CC, with the immersed area in the electrolyte being 1 cm × 1 cm, a 1 cm × 1 cm platinum piece as the counter electrode, and an Ag/AgCl electrode as the reference electrode, using a 3 M KCl solution as the electrolyte. A xenon lamp light source served as a simulated solar light source, capable of outputting light intensity equivalent to one sun, approximately 100 mW cm−2.
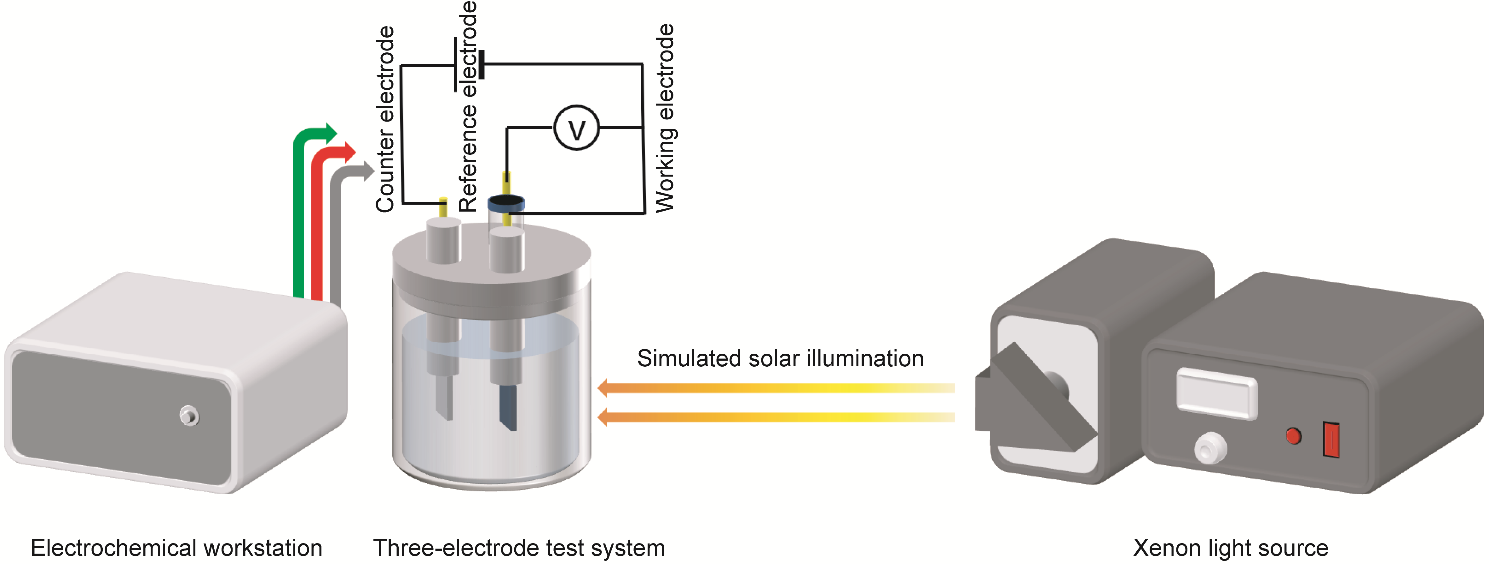
Figure 4. Schematic of the electrochemical testing setup
Initially, to verify the photoelectrochemical functionality of Cu-CAT, which is the generation of photo-induced electron/hole pairs under illumination that participate in electrochemical reactions before recombination, its time-current curve (referred to as the IT curve) was tested. By toggling the simulated light source on and off during the test, the changes in photocurrent were monitored. In Figure 5a, the photocurrent was calculated to be 8 µA. To eliminate any influence from the carbon cloth on the photocurrent, a control test was conducted as shown in Figure 5b. Electrodes were prepared by coating carbon nanotube films (CNT) with Cu-CAT powder; CC and CNT served as the blank control group. Under the same illumination, Cu-CAT@CNT exhibited a photocurrent of 78.1 µA, ten times higher than that of Cu-CAT@CC. The photocurrent of the control group differed by several orders of magnitude from that of Cu-CAT@CNT, with both carbon cloth and carbon nanotube films having negligible impact on photocurrent, thereby confirming the effective photo-electroconversion functionality of Cu-CAT.
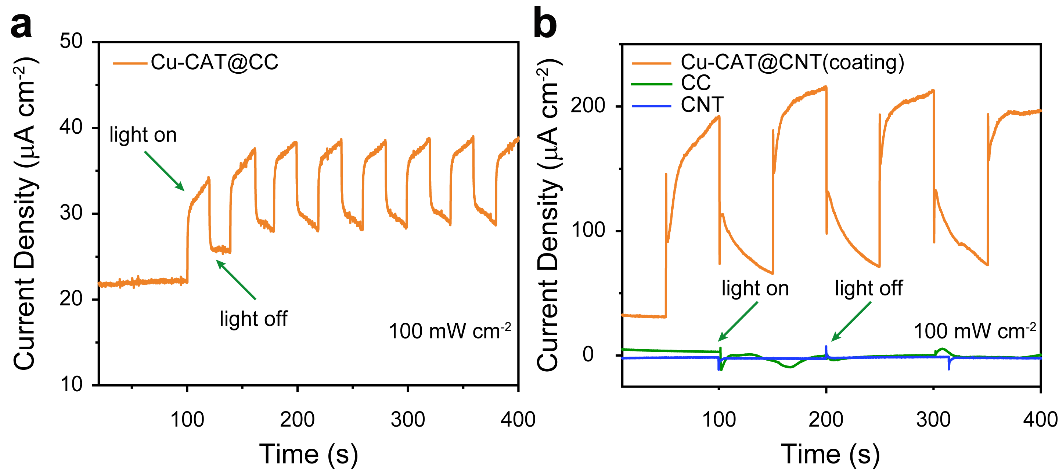
Figure 5. a. IT curve of Cu-CAT@CC; b. IT curve of the control group
Further testing of Cu-CAT@CC involved cyclic voltammetry (CV) and galvanostatic charge-discharge (GCD) curves under illuminated and non-illuminated conditions, as shown in Figures 6a and 6b. The current sweep rate was 50 mV s−1 with a voltage window of 0-0.7 V. The specific capacitance was calculated from the area under the CV curves before and after illumination, showing a 3.2% increase in capacitance under light. Similarly, in Figure 6b, at a current density of 1 mA cm−2, the specific capacitance of Cu-CAT@CC after illumination increased by 8%.
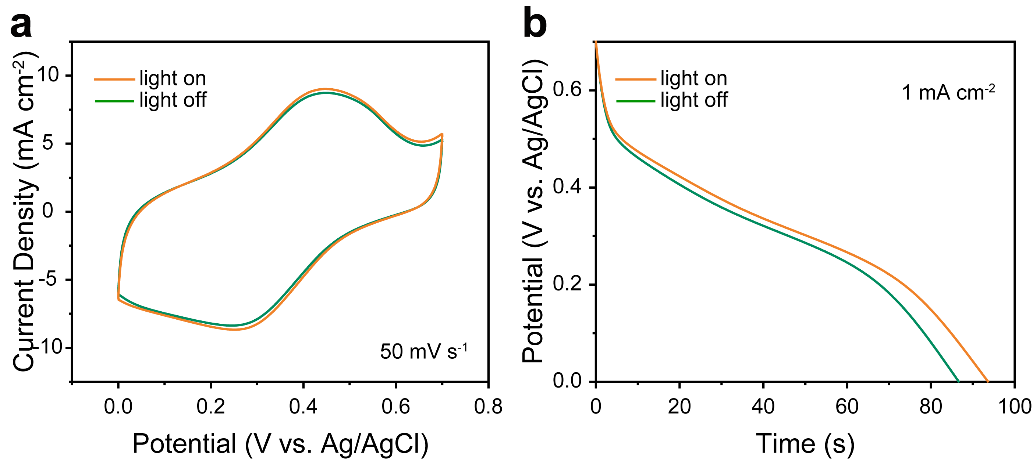
Figure 6. CV (a) and GCD (b) curves of Cu-CAT@CC
To verify the application of Cu-CAT@CC in an aqueous zinc-ion hybrid supercapacitor (denoted as Zn//Cu-CAT@CC), it was used as the photoactive cathode with a zinc sheet as the anode, and a 2 M ZnSO4 solution as the electrolyte, tested in a two-electrode system at the electrochemical workstation. In Figure 7a, the CV curve with a sweep speed of 50 mV s-1. The area increased under illumination compared to non-illuminated conditions, ultimately calculating a 7.9% increase in storage capacity. Figure 7b shows the GCD curve, where the current density was 1 mA cm−2 and the discharge time of the device significantly increased under illumination, with the first complete cycle discharge capacity reaching 0.162 mAh cm−2, an 89.1% increase compared to non-illuminated conditions.
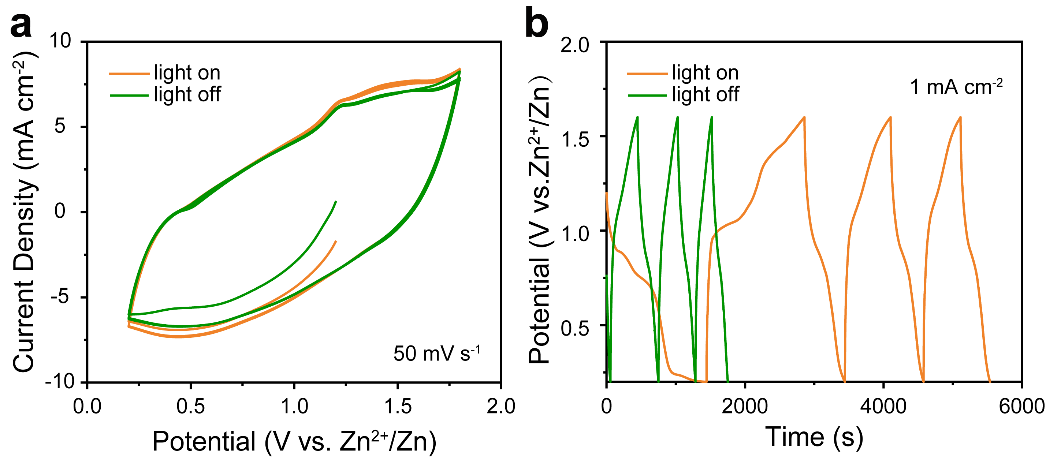
Figure 7. CV (a) and GCD (b) curves of Zn//Cu-CAT@CC
Electrochemical impedance spectroscopy (EIS) was also tested before and after light application, as shown in Figure 8. Under illumination, the device's charge transfer resistance further decreased, facilitating rapid intercalation/extraction of zinc ions within the pores of the Cu-CAT@CC electrode, thereby enhancing the device's kinetic performance.
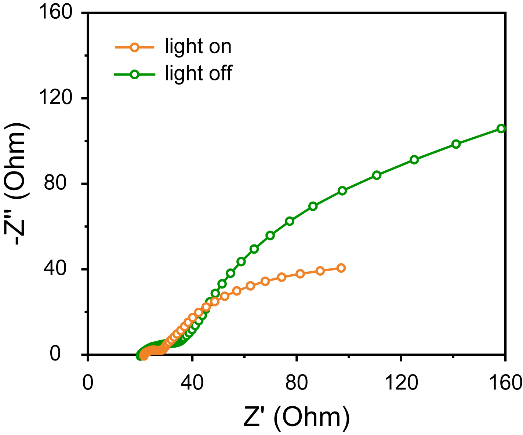
Figure 8. EIS curve of Zn//Cu-CAT@CC
4. Conclusion
In summary, this paper demonstrates the feasibility of using Cu-CAT nanorod arrays grown on carbon cloth (CC) surfaces via a hydrothermal method to create CC-CAT@CC electrodes. The structure was characterized using SEM, UV-vis, and UPS, which first verified its viability as a photoactive electrode. This Cu-CAT@CC was then used as the photoactive cathode to fabricate an aqueous zinc-ion hybrid supercapacitor, with its performance evaluated by testing CV, GCD, and EIS curves before and after illumination. Under a current density of 1 mA cm−2, the areal capacity under illumination reached 0.162 mAh cm−2, an 89.1% increase over the capacity without illumination. This not only opens up new directions in the application of Cu-CAT materials but also broadens the prospects for constructing aqueous zinc-ion hybrid supercapacitors and expanding the application of clean energy.



