1. Introduction
The paradigm of self-assembly is fundamentally inspired by observations in nature, wherein biological entities, including proteins, DNA, and cells, exhibit a propensity to spontaneously organize into structures that are functionally significant [1]. The meticulous examination of such natural phenomena has been instrumental in laying the foundational framework for the evolution of synthetic self-assembly methodologies, thereby elucidating the governing principles of spontaneous organizational structures. In the initial decades of the 20th century, the scientific fraternity embarked on a journey to unravel the principles underpinning self-assembly in synthetic systems. A seminal instance of this investigative endeavor was the comprehensive study of surfactants and micelles, elucidating the inherent capability of molecules to spontaneously coalesce into ordered configurations [2]. The mid-20th century heralded the rise of supramolecular chemistry, a pivotal field advancing self-assembly methodologies. Self-assembly is a native process. The process happens when individual components spontaneously organize into ordered structures at the nanoscale. Self-assembly can be classified into two main types: static and dynamic. In static self-assembly, the organization of the final structure may require energy, but it is stable once it is formed. Examples are lipid molecules forming oil droplets in water, four hemoglobin polypeptides forming a functional hemoglobin protein, and the combination of RNA and ribosomal proteins to form a functional ribosome [3]. However, the dynamic self-assembly process is not as stable as the previous one. It involves the continuous and reversible formation of structures in response to external stimuli. Most studies have been focused on static processes in self-assembly, while the study of dynamic self-assembly is still immature. So, the aim of this review is to furnish a comprehensive and integrative overview of the advancements realized in one of the most brunches of dynamic self-assembly, responsive self-assembly. The review endeavors to trace the evolutionary trajectory of these methodologies, probe into the mechanisms that underlie the responsive self-assembly of nanomaterials, and underscore the pioneering applications and prospective future developments in this vibrant and ever-evolving field.
2. Related work
The annals of scientific literature are replete with treatises on self-assembly, each echoing the collective curiosity and wonder of the scientific fraternity. The early forays into this domain were marked by a fervent desire to unravel the fundamental forces that beckon molecules into spontaneous congregations. The 1980s, for instance, were illuminated by Lehn’s trailblazing insights into molecular recognition processes, a magnum opus that would fetch him the coveted Nobel Prize in Chemistry in 1987 [4]. The baton of enquiry was then passed to luminaries like Whitesides and his peers, who delved deep into the subtle interplay of weak forces, such as van der Waals and hydrogen bonding, in sculpting the self-assembled masterpieces [5]. In a confluence of biology and technology, Mirkin and his team showcased the artistry of using DNA as a scaffold, guiding nanoparticles into intricate assemblies, and heralding a new era in nanostructure fabrication [6]. As the chronicles of self-assembly continue to be penned, each chapter adds depth, nuance, and perspective, yet the story is far from complete, with the promise of many more revelations on the horizon.
3. Mechanisms of Responsive Self-Assembly
Responsive self-assembly can be understood by first examining its foundational concepts: static self-assembly and dynamic self-assembly. In a static process, without external influences, the self-assembly of the structure is driven by energy minimization, while in a dynamic process, there are external influences and the self-assembling system adapts to its surroundings. Figures 1 and 2 are images indicating the difference between static self-assembly and dynamic self-assembly.
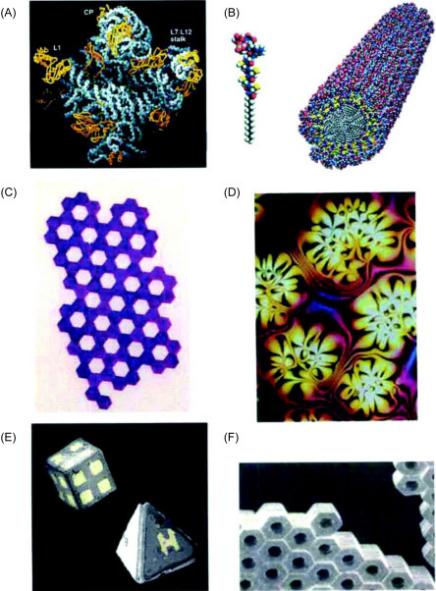
Figure 1. Examples of static self-assembly: (A) Crystal structure of a ribosome (B) Self-assembled peptide–amphiphile nanofibers (C) An array of millimeter-sized polymeric plates (D) A thin film of a nematic liquid crystal (E) Micrometer-sized metallic polyhedra folded from planar substrates (F) A three-dimensional aggregate of micrometer plates assembled by capillary forces [7]
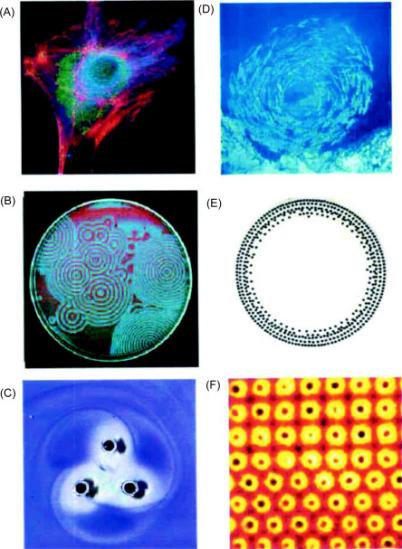
Figure 2. Examples of dynamic self-assembly: (A) An optical micrograph of a cell with fluorescently labeled cytoskeleton and nucleus (B) Reaction-diffusion waves in a Belousov–Zabatinski reaction (C) A simple aggregate of 3-mm sized, rotating, magnetized disks interacting with one another via vortex–vortex interactions (D) A school of fish (E) Concentric rings that formed by charged metallic beads 1 mm in diameter rolling in circular paths on a dielectric support (F) Convection cells formed above a micropatterned metallic support [7]
Responsive self-assembly refers to the process by which individual nanoparticles spontaneously organize into ordered structures in response to external stimuli. This process follows specific patterns based on many factors, including the nature of the external stimulus and the properties of the nanomaterials. Common external stimuli include temperature, pH, light, magnetic fields, chemical agents, etc. Inherent properties of nanomaterials are their molecular interactions, mechanical properties, size, shape, charge, hydrophobicity, etc. These factors are crucial in determining the stability and reversibility of the responsive self-assembly process.
Theoretically, temperature impacts the self-assembly process by changing the Gibbs free energy change, which is calculated by entropy, enthalpy, and temperature. The Gibbs free energy change influences spontaneity in that the negative Gibbs free energy change indicates a spontaneous process.
Thermo-responsive Materials are a class of materials that undergo reversible changes in their physical properties, such as phase transition, in response to temperature change. Some materials are soluble in water when below the Lower Critical Solution Temperature (LCST) and are insoluble above the LCST. For example, thermos-responsive polymers, such as poly(N-isopropylacrylamide) (PNIPAAm), have been used to control responsive self-assembly. Upper Critical Solution Temperature (UCST) is another temperature boundary whose property is exactly opposite to LCST. These transitions of solubility can be utilized in thermos-responsive self-assembly in various concentrations. Figure 3 gives an example of the use of LCST and UCST. In the LCST behavior, the phase change occurs by increasing the temperature, while in the UCST behavior, the phase change is in the opposite direction.
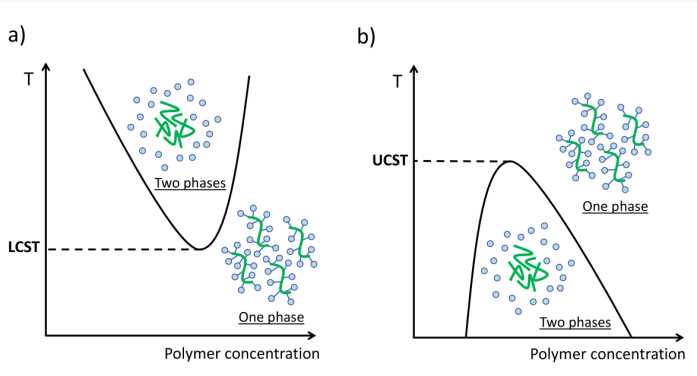
Figure 3. Phase diagram in the case of LCST and UCST behaviors [8]
Light is a form of electromagnetic radiation with properties such as wavelength, frequency, intensity, etc. These properties are changeable to induce specific responses in photo-responsive nanomaterials such as azobenzene and diarylethene. The excited state conformation of some light-activated molecules is another method to employ light as a trigger. When a molecule absorbs a photon of light, one of its electrons is promoted from a lower energy orbital to a higher energy orbital resulting in a transition from the ground state to the excited state which is the driving force of the responsive self-assembly process.
Besides external stimuli, there are many different forms of molecular interactions that can affect responsive self-assembly, such as hydrogen bonding, Van der Waals forces, electrostatic interactions, etc. The bonding type of the nanomaterials influences the final organization. Of these molecular interactions, hydrogen bonding is the strongest one. A hydrogen bond is a dipole-dipole interaction between a hydrogen atom bonded to a highly electronegative atom, called a donor, and another electronegative atom, called an acceptor.
The rate of stimulus is also an important factor in the responsive self-assembly process. A slow induction of stimulus leads to the formation of well-defined structures, while a rapid induction causes the formation of irregular structures.
4. Experiments and Application
Thermo-responsive polymers
Thermo-responsive polymers have been used in multiple fields such as cell culture, chromatography, colloidal stabilisers, and enhanced oil recovery (EOR) [8].
Cell culture is essential in biology, allowing cells to grow under controlled settings. Using thermo-responsive polymers like PNIPAAm in culture dishes enables easy cell detachment by just reducing the temperature. This innovation aids in studying cell biochemistry, drug testing, and other research areas.
Chromatography separates compounds based on their interactions with stationary and mobile phases. Different compounds move at varied rates due to their affinities for the stationary phase. Thermo-responsive polymers, like PNIPAAm, enhance separation based on hydrophobicity and charge, effectively isolating molecules like steroids and protein complexes.
Colloidal stabilization prevents particle aggregation in products like paints and pharmaceuticals. Thermo-responsive polymers offer temperature-controlled stabilization and enhance the management of colloidal system stability in products such as cosmetics and medicines.
Thermo-responsive polymers, when injected into oil reservoirs, can also alter their viscosity to boost oil displacement, enhancing oil recovery during EOR processes.
The field is still evolving, and there is ongoing research to explore new possibilities and improve the performance of these polymers in various applications.
Photo-Controlled Self-Assembly of Nanoparticles
In the paper “Photo-Controlled Self-Assembly of Nanoparticles: A Promising Strategy for Development of Novel Structures,” researchers explored photo-responsive nanomaterials. They demonstrated the precision and rapid control of light stimulus in the development of new functional materials [9].
The research highlights two primary techniques to achieve this light-driven self-assembly:
The Photochemical Process: This method utilizes photochromic molecules, notably azobenzene, spiropyran, and diarylethene (DAE), to alter nanoparticles. The driving force for the self-assembly process is the structure changes of these photo-responsive molecules in the presence of light.
The Photophysical Process: Unlike its photochemical counterpart, this method triggers self-assembly by leveraging the excited state conformation, bypassing the need for conventional light-sensitive molecules. Molecules like polyphenylthiobenzene derivatives exhibit this excited state conformation upon light exposure.
Each of these processes comes with its own set of pros and cons. The photochemical process boasts greater reversibility due to specific chemical reactions. However, its dependence on UV light and susceptibility to side reactions can pose challenges, especially in varying environments. In contrast, the photophysical process, while more adaptable for commercial applications since it doesn’t hinge on specific chemical reactions, is still in its infancy. Researchers are grappling with the task of creating self-assembling molecular systems powered by spatial conformations. The concept of light-controlled self-assembly in nanoparticles holds immense promise. It paves the way for the invention of innovative nanostructures, offering meticulous control and resulting in advanced materials endowed with distinct properties and functions. Such a technique can revolutionize areas like drug delivery, where the assembly and disassembly of certain drugs can be governed by light, or in catalysis, enhancing efficiency and precision.
5. Discussion
Thermo-responsive self-assembly and photo-controlled self-assembly are both great in terms of controllability since the processes are reversible in response to temperature and light change. By changing the external stimuli, the self-assemble and self-disassembly processes can both be readily approached. Reversibility is vital in both advanced drug delivery systems and smart surfaces. The ability to control the permeability of the surface allows for the controlled release of drugs during drug delivery cycles [10]. They are also both environmentally friendly because the processes only require temperature and light as external stimuli instead of chemical additives. However, many aspects of the two processes are still challenging. The number of thermo-responsive self-assembly nanomaterials, especially UCST-based polymers, is limited due to the limited temperature range and their sensitivity to environmental conditions. More importantly, the production of thermoresponsive polymers can be expensive. So, more research studies on thermoresponsive polymers are needed to spread their applications. Moreover, the efficiency and specificity of photo-controlled self-assembly nanomaterials can be influenced by the wavelength, intensity, and duration of light exposure, which limit their application scenarios. In recent years, researchers not only keep overcoming the challenges associated with their properties, costs, scalabilities, and sensitivities but also explore their potential developments in advanced drug delivery systems, smart surfaces, etc.
6. Conclusion
Thermo-responsive and photo-controlled self-assembly processes have emerged as powerful tools in nanotechnology. Their environmentally friendly properties and high controllability, which only rely on temperature or light, make them outstanding compared to traditional methods that often depend on chemical additives. However, the limited variety of thermo-responsive materials and the low efficiency of photo-controlled self-assembly are still challenging. Enhancing the diversity of thermo-responsive materials and refining the photo-controlled self-assembly process to be less dependent on precise light conditions will be the main focus of future research. This review could benefit from a deeper exploration of interdisciplinary studies on responsive self-assembly that combine both thermo-responsive and photo-responsive advantages and minimise their imperfections. Additionally, discussion of more real-world case studies could provide more practical insights into the potential and limitations of these processes.
Acknowledgment
Firstly, I would like to show my deepest gratitude to my teachers and professors in the research project, Nano-system analysis of polymer nanomaterials, condensed matter physics and self-assembly materials, cosmetics, blood, and other soft substances. They provided me with valuable guidance in many stages of the writing of this thesis. Further, I would like to thank all my friends and parents for their encouragement and support. Without all their enlightening instruction and impressive kindness, I couldn’t have completed my thesis.



