1. Introduction
Assemble by silicon-containing polymers while macromolecular siloxanes as the main structure particle is called silicon-based material and polymers [1]. Materials with one dimension between 1 and 100 nm are referred to as nanomaterials [2]. Two different types of batteries used lithium: lithium metal and lithium-ion batteries; the main difference is the cell type: lithium metal batteries cannot be rechargeable because of the primary cell construction while lithium-ion batteries can rechargeable and repeatedly used is due to the secondary cell construction. A type of rechargeable battery that has lithium ions that can transfer between the cathode and anode when the battery is charged and discharged is called a lithium-ion battery. The use of lithium inside batteries was created by Whittingham in 1976 which is a US engineer who works in an oil company [3]. Lithium-ion batteries for a variety of consumers such as self-phones were introduced in the 1990s [3]. Before the lithium-ion batteries create the researcher are focusing on lithium metal batteries: use metallic lithium as the anode material. Because Li dendrites growth in the Lithium metal battery could cause a serious safety issue so the research has standstill since the 1980s.
The place between the cathode and anode is used to stores electrolytes while a separator is inside. The function of the separator is to prevent electrons to move between the anode and cathode followed by only allowing lithium ion to pass. During discharging the lithium and the electron move from the anode and arrived at the cathode at the same time and vice versa. However as mentioned above that the electrons cannot cross the separator, so they move in the external circuit. Otherwise, because the lithium-ion has a positive charge when leaving the cathode that it will attract the solvent to form a shell that surrounds the lithium-ion is called a solvation shell. However, during the first-time charge and discharge the solid electrolyte interphase (SEI) layer has appeared. This layer can make sure the continuation of the electrochemical reactions while it can prevent further electrolyte decomposition by preventing electrons and allowing lithium ion to cross [4]. On the other hand, some capacity of the battery will lose because of that. The area of battery use has increased a lot in the last few decades especially in the last decade, such as the numbers and types of mobile phones, tablets, and laptops.
Afterwards, the shipment volume of iPhone instrument increased rapidly from 2010 (50 M) to 2015 (approximately 220 M) and have waved a little from 2015 (220 M) to 2020 (approximately 200 M) [5] which directly shows the requirement and need for lithium batteries were also raised at the same time in the last decade, which asked the technology to create more efficient batteries, for example, high charging speed and more capacity on mobile phones and tablets and control the weight at the same time. Researchers are trying to discover new materials for batteries Honor has launched a silicon carbon battery and Xiaomi’s silicon Oxygen Negative Battery both improved the material and structure of the anode. These technologies helped them to fit higher capacity batteries in mobile phones.
This research will analyse how silicon-based material improved the effectiveness of lithium-ion batteries in different dimensions such as capacity, charging speed and reduce weight and thickness. Afterwards, this project will first discuss the problem of traditional batteries and the solved by predecessor researchers while new types of silicon-based anode batteries and the technology with examples such as Tesla and Honor secondly. Finally, is to discuss the benefit of using silicon-based nanomaterial.
2. Why do predecessor researchers stopped the discover of lithium metal batteries?
As mentioned above, lithium metal batteries use metallic lithium as the anode material. Lightweight, contain huge amount of energy, and non-flammable are the benefit of it. However, the research and discover of lithium metal standstill during 1980s due to the safety issue such as Li dendrites growth inside the batteries may cause fire. The results show that short circuit can be caused by the open of nanoscopic fissures in the battery material which caused by bending, twisting or just modest indentation. Stress also can cause failure of the battery even some impurities such as dust in manufacturing [6]. This has caused by ask the manufacture ability and both inside and outside material strength into a very high level and will lead sharp increase of cost. The increase of cost may can be accepted in some wealth company or department but it’s unacceptable in most place nowadays especially in smartphone market. Because the safety and stabilization are not suitable, and the risk of accident is too high for mobile devices. According to some research paper, the energy density can significantly increase due to use metallic lithium in electrodes because of its potential. However, as shown in Figure 1, the performance can be degraded and may lead to short circuits due to the formation of lithium dendrites on the metal surface [7].
As mentioned above, there is a separator inside the electrolyte of battery which only allow specific ions pass through. In normal times, the current is not directly cross between cathode and anode instead of ion moving. However, the short circuit happened after the dendrites growth until the separator is broken or even, they reach the cathode. Even though, some scholars made efforts to stop of limit the growth of dendrites as much as possible or change into a new type of batteries. Two type of lithium deposits are on the right-hand side: mossy type on the top while dendritic type on the bottom while on the left-hand side the images show the ceramic material layer and it’s blocking effect to limit the growth of mossy [6]. Mossy is relatively easily to control because they grow up at a low speed from the root while dendritic is only grow in their end which means its reason that caused most of the problems. By adding a layer that used for separating that made by nanoporous ceramic material can block the growth as the mossy structure from the root [6].
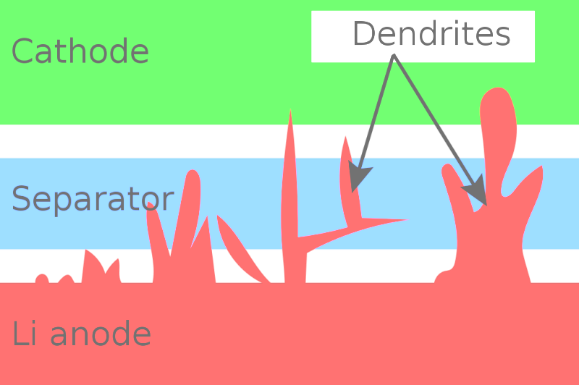
Figure 1. How does the dendrites broke the batteries from inside [7].
3. Application of silicon-based material in batteries
3.1. Why need to improve the batteries and limitations
There are billions of people who use mobile devices such as tablets, phones and laptops throughout the world and the requirement for battery life and charging time continues increasing as time goes on. In today’s fast-paced life consumers are asking for longer battery life and faster charging time to deal with long-distance journeys and fewer break times, on top of that some manufacturers are discovering technology on batteries. In early times Android phone companies decided to use high-volume batteries in mobile phones while Apple used more advanced chips to reduce power consumption and slowly increase the battery capacity.
As shown in Table 1, after a few years the battery life has increased a lot but unfortunately, the weight has also increased into an unacceptable level such as the iPhone 14 Pro Max weighing 240g. However, the company must find a battery with both high capacity and a lower or same weight, so they chose silicon. The reason for using silicon to replace graphite is because it can shorten the charging time and have more battery range followed by lighter weight. According to other researchers, silicon can contain 10 times more lithium ions than graphite and EV’s could go 50% further [8]. Afterwards, this improvement is more obvious on mobile phones, tablets, and other small cell electronic devices, it can make consumers feel directly that the battery life and charging time have improved.
Table 1. Analysis of the battery capacity of different devices [9].
Devices | Battery Capacity |
Honor 10 (2018) | 3400 mAh |
Honor 90 (2023) | 5000 mAh |
iPhone Xs Max (2018) | 3174 mAh |
iPhone 14 Pro Max (2023) | 4323 mAh |
3.2. Honor’s new battery technology
Honor launched the world's first silicon carbon battery in MWC on February 27th, 2023, according to Honor’s CEO George Zhao shown during the event this technology can increase the energy density by 12.8% compared to graphite battery, which means it can contain the same capacity in smaller areas or contain more in the same area. Some Chinese technic press said that Honor uses the porous carbon skeleton plus nano-silicon in-situ vapour deposition technology, and through graphite doping with silicon. The Honor Magic 5 Pro has a 5100 mAh battery capacity instead of 5450 mAh after using this technology and it will have 240% more capacity in a 3.5 V low voltage situation compared to standard graphite as shown by the following image [10]. Afterwards, as mentioned above the silicon can lighter the weight of the battery at the same capacity and the data from their official website that the Honor Magic 5 Pro (5450 mAh) weight 219 g which is a little bit heavier than Huawei Mate 50 Pro (4700 mAh) which weight 205 g, but it contains more capacity which also bigger and lighter than iPhone 14 Pro Max (4323 mAh). Although the use of silicon in an anode also can improve the charging speed Honor still chose a medium charging speed after considering the heat during charging time will damage the electrolyte and later reduce the capacity.
3.3. Xiaomi’s new battery technology
Honor is not the only mobile phone manufacturer that has discovered new battery technology. Xiaomi has already launched a silicon-oxygen anode battery in 2021 during the Xiaomi 11 launch event. Silicon-oxygen battery technology is used in electric cars by adding nano silicon material into the anode to have a bigger capacity and charging speed and that is why Xiaomi announced on the poster: ‘It can make mobile phone battery thinner and charging faster’. It has the same benefit as Honor’s new battery: the ratio between space and capacity, however, as reported by the China Battery Industry Association the safety of the three battery anode materials is: carbon material>silicon material>silicon oxide material, and the silicon battery is easy to bulge. The cover material and other aspects must have a higher requirement during manufacturing and lead to the cost increase at the same time. On the other hand, carbon material has the smallest expansion coefficient (about 10%) but the energy density is only 372 mAh per gram while silicon can reach approximately 4200 mAh per gram but with a 300% high expansion coefficient while silicon oxide gets 2615 mAh per gram with about 160% expansion coefficient. Why Xiaomi did not use 120-watt super-fast charging on the Xiaomi 11 Pro and Ultra as they used to do on the Xiaomi 10 series instead of only 67-watt charging to ensure security.
4. Conclusion
This paper has discussed the failure of lithium metallic batteries discovered at first but lacks any examples, including experiments in the 1980s or even a failed example. Afterwards, according to Honor and Xiaomi’s new silicon-based battery, there is nothing information showing how they do inside the battery Perhaps it is classified, but it is less authoritative and accurate without the inside structure, only can use the information that they show on their launch event. Silicon-based nanomaterial can improve the performance of batteries in capacity, weight and charging speed because it has 10 times more lithium-ion capacity compared to graphite batteries. The reason that lithium metallic batteries failed and stopped discover is they are very easy to have a short circuit by bending, twisting or just modest indentation. Both electric vehicle and mobile phone companies are using silicon to improve their battery life, silicon carbon batteries and silicon-oxygen anode batteries.



