1. Introduction
For left and right hands, the two hands are mirror images that do not overlap. In addition, this phenomenon can be seen everywhere in nature. This phenomenon, in which an object cannot superimpose its mirror image, is called chirality. Chirality is one of the fundamental properties of nature. Therefore, in nature, most molecules contain chirality. For example, amino acids, which are essential for human life, are in L-configuration, and sugar, the main energy substance, is divided into D-configuration and L-configuration [1].
The difference in chirality can influence the properties of the molecule. In the human body, drug molecules perform pharmacological actions by binding to receptors. These receptor molecules have specific structures, so for different chirality drug molecules, the binding situation will be different, and the biological activity in the human body will also be different. The differences caused by this chirality range from ineffectiveness to toxicity. For example, in the anti-rheumatic drug penicillamine, the D-configuration is nontoxic, and the L-configuration is toxic [2].
Therefore, research on chiral drugs has always been the focus of research on new drugs all over the world. The synthesis of chiral drugs is also a frontier and a hot issue in drug research. In the 1960s, research on asymmetric catalytic synthesis reactions began. So far, the applications in chiral drug synthesis include asymmetric catalytic hydrogenation, asymmetric catalytic oxidation, asymmetric catalytic cyclopropanation, asymmetric catalytic carbonyl reduction, asymmetric catalytic carbonyl synthesis, asymmetric double bond transfer synthesis reactions, and asymmetric organocatalysis. Among them, the synthesis of asymmetric organocatalytic chiral drugs started relatively late compared to other asymmetric catalysis methods, so the number of papers on asymmetric organocatalytic chiral drugs is very small.
Metal and enzymatic catalysts have long been used in the field of chemistry, but for asymmetric synthesis, most of these catalysts do not have the corresponding capabilities. In 2000, Benjamin List and David W. C. MacMillan developed asymmetric organocatalysts, which brought asymmetric organocatalysis to a climax and entered its golden development period [3]. Organocatalysts are the third class of catalysts, and asymmetric organocatalysis has made great contributions to the synthesis of chiral small organic molecules. Asymmetric organocatalysis can increase the content of the ideal enantiomer and improve the synthesis efficiency through induction [11]. In addition, this technology has also had a huge impact on asymmetric reactions such as Mannich reaction, Michael addition reaction, and Diels-Alder reaction, and has also led to the emergence of a series of new organic catalysts in chiral chemicals, drugs, and materials. It has made a non-negligible contribution to the industrial application of chiral chemicals, drugs, materials, pesticides, etc. [3].
In this paper, the research of asymmetric organocatalysis in individual chiral drugs is summarized, and its prospects for drug research are also summarized. It is hoped that the examples of asymmetric organocatalysis in chiral drugs will be generalized, and the specific examples of asymmetric organocatalysis in chiral drugs will be provided later.
2. Application of chemical synthesis in chiral drugs
2.1. Antidepressant chiral fluoxetine
Depression is the fourth largest disease in the world, and the incidence trend is currently younger. It is mainly characterized by continuous long-term depression as the main clinical feature. In severe cases, it can lead to self-harm or suicidal behavior. Fluoxetine is a common psychiatric treatment drug. It can selectively inhibit 5-HT transporters, block the reuptake of 5-HT by the presynaptic membrane, prolong the effect of 5-HT, and achieve antidepressant effects. The main efficacy of the (R) configuration of fluoxetine is to treat depression, and the (S) configuration of fluoxetine can prevent migraines [4]. In addition, compared with (S) configuration fluoxetine, (R) configuration fluoxetine has fewer side effects due to its short half-life. Therefore, the separation of chiral fluoxetine is necessary to achieve the target efficacy. The initial methods to obtain the chiral fluoxetine configuration include chemical resolution, enzymatic resolution, chiral prosthetic group induction, asymmetric epoxidation, asymmetric addition, asymmetric reduction, and enzymatic asymmetric synthesis [4]. These approaches have limitations due to the cost of catalysts, the stringent conditions of the reaction, and the hazardous nature of chemical starting materials [5].
In recent years, some progress has also been made in the preparation of fluoxetine by asymmetric organocatalysis. Studies have shown that the organocatalytic asymmetric epoxidation of α, β-unsaturated aldehydes has good yield and high enantioselectivity, so the organocatalytic asymmetric synthesis of fluoxetine based on cinnamaldehyde epoxidation is feasible [5].
This reaction catalyzes the reaction of cinnamaldehyde and hydrogen peroxide through the organic catalyst (S)-2- [diphenyl (triethylsiloxy) methyl] pyrrolidine, which undergoes a series of transformations to form (S) Configuration of fluoxetine (Figure. 1). In the reaction, the chiral alcohol is determined by organocatalysis, so the final configuration of fluoxetine is also determined. This is very beneficial for the preparation of chiral fluoxetine [5].
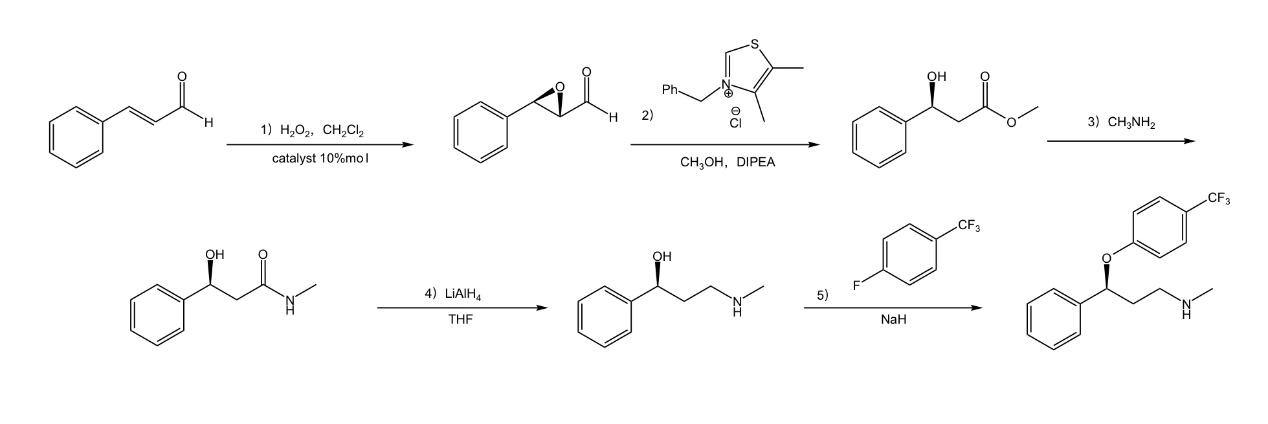
Figure 1. The synthetic route of (S)-fluoxetine [5].
2.2. Pregabalin
Pregabalin, also known as ((S)-3-aminomethyl-5-methylhexanoic acid), was developed by Pfizer. Pregabalin acts on the central nervous system and has contributed significantly to the treatment of epilepsy, neuropathic pain, and fibromyalgia. In the preparation of pregabalin, γ-amino acids have been used many times in the past. For example, Pfizer's production of pregabalin is based on (S)-mandelic acid crystallization to distinguish racemic γ-amino acids [6] [8], followed by a synthetic sequence based on a bisphosphine complex-catalyzed asymmetric hydrogenation step [7] [8].
Recently, a new and economical method has been developed. According to literature reports, inexpensive chemicals such as isovaleraldehyde 1 and nitromethane 2 are used as synthetic raw materials to first synthesize nitroalkene 3, and through the addition of dimethyl malonate 4, through asymmetric organic catalysis, the final product is γ-amino acid intermediate 5, the final synthesis of pregabalin (Figure. 2) [8].
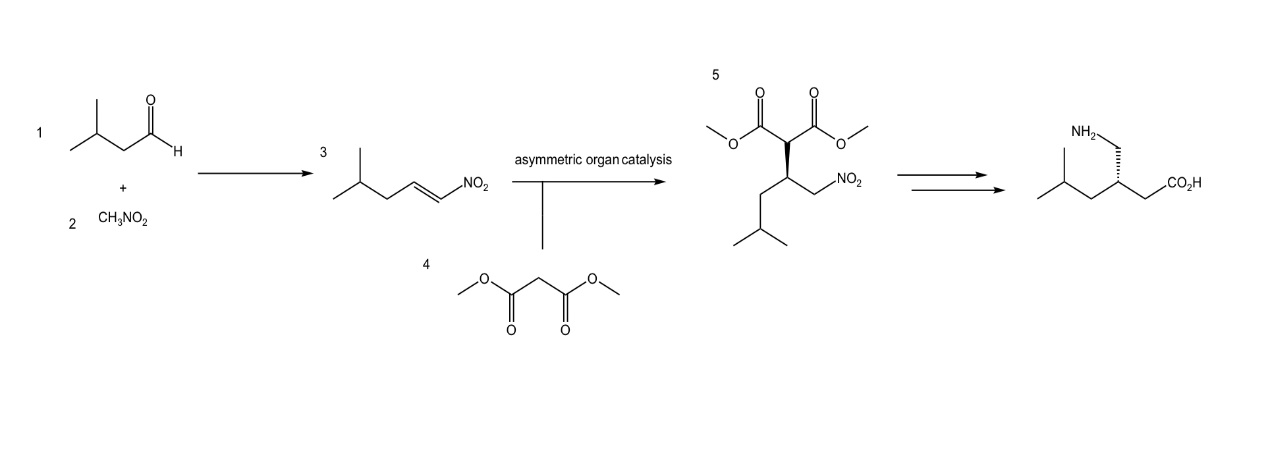
Figure 2. The synthetic route of Pregabalin [8].
In asymmetric organocatalytic steps, catalyst selection is a challenge. The ee values of epi-9-deoxy-9-amino-Cinchona-derived squaramides were greater than 80%, indicating a high selectivity of -amino acid adducts under these conditions. For quinidine catalysts, it was ideal to have two other derivatizing reagents (quinidine reagent 6 and quinidine reagent 7) (Figure. 3), both of which had high conversions in the reaction with nitroalkenes of 98% and ee values of 91% [8]. However, quinidine catalyst 7 exhibited better reactivity when using unpurified nitroalkenes [8].
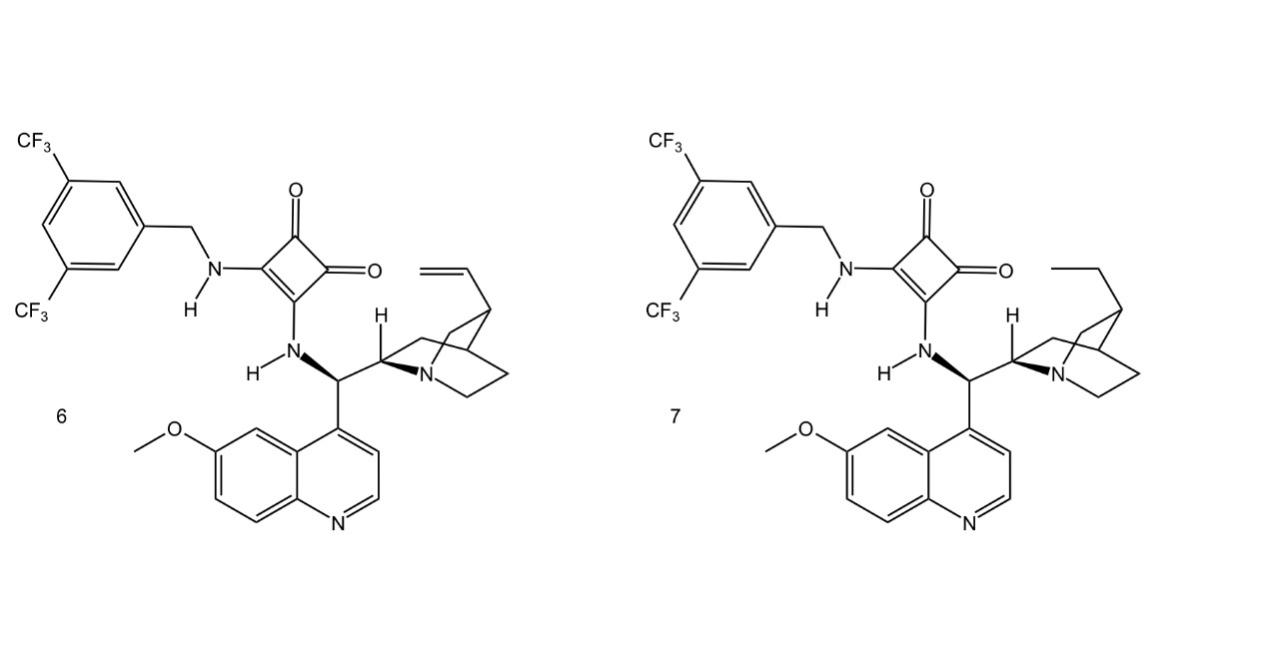
Figure 3. Two derivatizing reagents of quinidine reagent [8].
This route worked efficiently at room temperature. However, catalyst synthesis and recovery are issues that must be considered. In addition, for the synthesis of nitroalkenes, although four different schemes were mentioned in the literature, they all had shortcomings that needed to be overcome. Therefore, this asymmetric organocatalytic route is still challenging for process production.
2.3. Dapoxetine
Dapoxetine was first launched in Europe in 2009. It is a rapidly acting and metabolized selective serotonin uptake inhibitor (SSRI). The main role of dapoxetine is to regulate the content of neurotransmitters such as serotonin in the libido center of the brain, thereby reducing reactivity and delaying the time for men to reach orgasm [9]. Premature ejaculation is a common sexual dysfunction, but there is no clear definition. In the clinical drug treatment of premature ejaculation, selective serotonin absorption inhibitors are usually used [9]. Therefore, dapoxetine is used to treat premature ejaculation in men.
The route of asymmetric organocatalytic preparation of dapoxetine is through the Aza-Michael reaction of cinnamaldehyde and Cbz-protected hydroxylamine to form addition product 1, followed by ring opening to form product 2, then a series of steps to synthesize the S-configuration of dapoxetine (Figure. 4) [9]. The organocatalyst in the process is [diphenyl-[(2S)-pyrrolidin-2-yl] methoxy]-trimethylsilane (Figure. 5) [9].
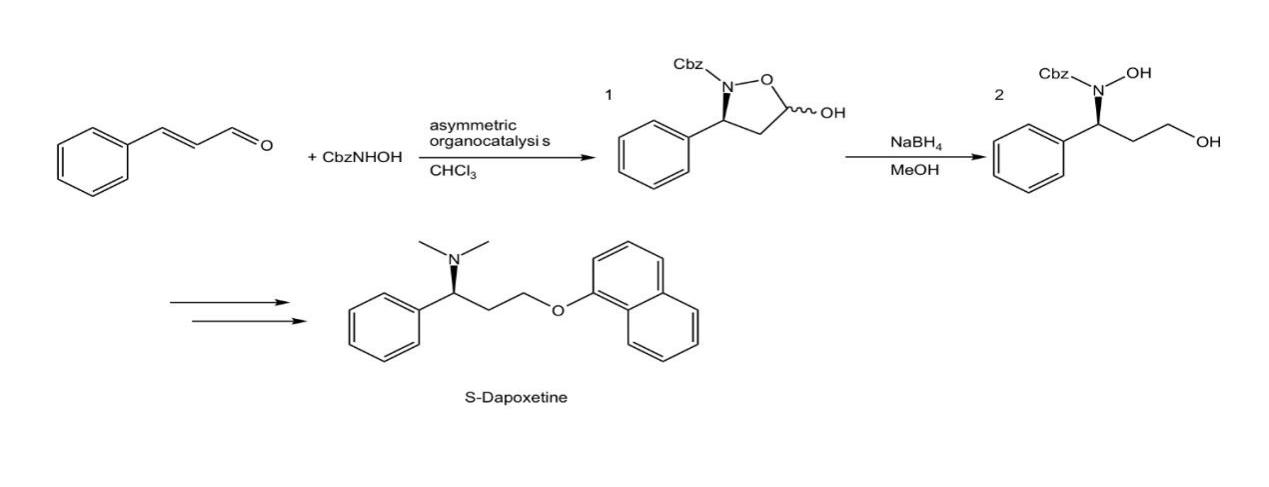
Figure 4. The route of asymmetric organocatalytic preparation of dapoxetine.
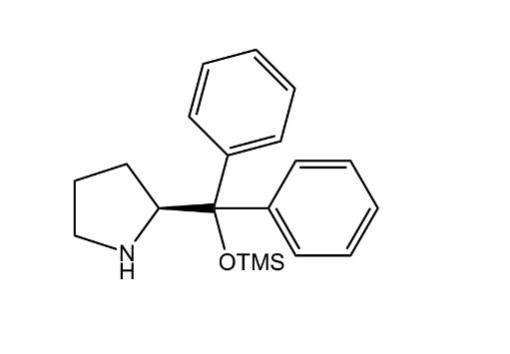
Figure 5. [diphenyl-[(2S)-pyrrolidin-2-yl] methoxy]-trimethylsilane.
The way to synthesize dapoxetine is still based on the racemate synthesis due to the complicated process of asymmetric organic catalytic synthesis.
2.4. Spirocyclic oxindole scaffolds
The spirocyclic oxindole scaffold is an important pharmaceutical compound, and this scaffold has a very wide range of drug activities. According to the authors, the asymmetric synthesis of spirocyclic oxindole-tetrahydrothiopyran scaffolds has not been reported [10]. The authors developed a method for organocatalytic asymmetric synthesis of it (Figure. 6).
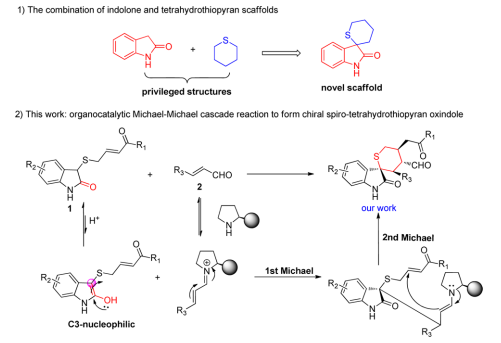
Figure 6. Organocatalytic Enantioselective Access to Chiral Spiro-tetrahydrothiopyran−Oxindole [10 ].
This was a newly designed Michael-Michael cascade process using a novel substrate 1 with nucleophilic oxindole-C3 as the Michael donor and α, β unsaturated ester as the Michael acceptor [10]. The authors found that oxindole-spiro-tetrahydrothiopyran gave relatively good yields when combined with diphenylprolinylsilyl ether as a catalyst and PhCO2H as an additive in DCM [10]. Under these conditions, the authors explored the reaction of various 3-substituted Indolin-2-one derivatives with α, β-unsaturated aldehydes, and the results showed mostly moderate and good yields with excellent corresponding selectivity [10].
In addition, the authors used the MTT assay to determine the in vitro antitumor properties of these oxindole-spiro-tetrahydrothiopyran derivatives. The results showed that this class of derivatives has desirable inhibitory activity against cancer cell lines (A549 lung cancer cells, HCT116 colon cancer cells, and MDA-MB-231 breast cancer cells) [10]. After that, the authors further studied the spirooxindole scaffold with the best test results, and the results showed that this spirooxindole scaffold could interact with the hot spot on MDM2 and inhibit the p53-MDM2 interaction in cancer cells [10].
The organocatalytic Michael-Michael cascade reaction developed by the authors was successful, and the results also demonstrated a novel spirooxindole scaffold with desirable yields, excellent enantioselectivity and antitumor activity, showing that it has good potential for antitumor drugs [10].
3. Discussion and Outlook
For the first three chiral drugs summarized in this paper, they have all been developed and put into production, and their production processes have basically been finalized. Asymmetric organocatalytic syntheses showed good results, but they were all small-scale and still challenging for large-scale production. For the fourth generalized chiral drug, asymmetric organocatalysis was a good example, showing that this technology is very feasible for the design and synthesis of new chiral drugs. Therefore, asymmetric organocatalysis has great potential in the future synthesis of new drugs.
In the past two decades, asymmetric organic catalysis technology has developed rapidly, gradually forming a sound theoretical basis and practical experience, breaking people's inherent thinking about catalysts and driving innovation ability [11]. The success of this technology is often accompanied by problems such as catalyst cost, efficiency and purification of the product [3]. Therefore, for organocatalysis and enzymatic catalysis, there have also been many explorations in the combination of the three catalytic methods of metal catalysis and will be more in the future [3].
In 2021, German organic chemist Benjamin List and American organic chemist David W. C. MacMillan were awarded the Nobel Prize in Chemistry by the Royal Swedish Academy of Sciences for their outstanding contributions to the study of asymmetric organocatalysis [12]. This also confirms that asymmetric organocatalysis is an important research field. In addition, this will also promote the further development of asymmetric organocatalysis.
In the future, asymmetric organocatalysis will make further contributions, such as providing green, efficient and safe synthetic processes for innovative drug research and development, or through this innovative catalytic mechanism, assisting the discovery of potential drugs, thereby creating a more comprehensive potential drug molecule, promote the further development of medicinal chemistry [11], provide possible therapeutic drugs for rare diseases, and reduce the fatality rate of rare diseases.
4. Conclusion
Asymmetric organocatalysis plays a huge role in promoting the synthesis of chiral drugs and has great potential in the development of new drugs. At present, it has achieved certain results, such as in the synthesis of pregabalin, fluoxetine, dapoxetine, and spirocyclic oxindole scaffolds. Although asymmetric organocatalysis has considerable expected effects on the synthesis of chiral drugs, there are still some shortcomings in its research. For example, these approaches are carried out under laboratory or small-scale conditions and are relatively complex for industrial synthesis. Second, the cost of experimental materials, recycling and pollution issues in asymmetric organocatalytic synthesis pathways are also to be considered.
In the future, further research can be done on organocatalysts in the asymmetric organocatalytic synthesis of chiral drugs. For the selection of organic catalysts, a variety of organic reagents can be used to compare small-scale experiments, and the ideal organic catalysts can be selected. Or for an ideal organic catalyst, synthesize its derivatives, and select the most ideal organic catalyst through experiments. In addition, the related synthesis process should also be studied, and the efficient asymmetric organic catalytic synthesis route should be improved in terms of process, reducing the cost of synthetic raw materials, improving the recovery efficiency, and expanding from the small-scale laboratory to the industrial aspect.



