1. Introduction
Rabies is an acute zoonotic infectious disease that is caused by viruses of the rabies genus. After the onset of the disease, it usually leads to acute encephalitis or meningitis, with a fatality rate of almost 100% [1]. Rabies is also known as hydrophobia. Rabies infects almost all warm-blooded animals, with its main hosts being dogs, cats, bats and wild carnivores. The rabies virus mainly exists in the central nervous system of diseased animals, such as the medulla oblongata, cerebral cortex, cerebellum and spinal cord. Viruses are mainly transmitted through the respiratory and digestive tracts. For instance, sick animals often carry viruses or bacteria when coughing, sneezing or having a runny nose, and form infectious droplets in the air to spread diseases. The saliva of sick animals also contains viruses. When pets kiss, lick, bite or scratch their owners, the virus enters the human body through saliva, causing infection. If people accidentally consume food or water contaminated by pets, diseases can be spread. After a person falls ill, their central nervous system is damaged. They are in a state of madness, with an extremely frightened expression, and eventually die due to respiratory and heart failure. The extremely high mortality rate of rabies has kept some people injured by dogs and cats in a state of long-term worry. It may even develop into obsessive-compulsive disorder and rabies hysteria, seriously affecting their normal work and life. After the outbreak of rabies, rabies patients and rabid animals exhibit behaviors of attacking others [2].
Due to the insufficient understanding of the basic mechanisms of nerve damage and functional disorders caused by the rabies virus, there is still no effective treatment for human rabies at present. The WHO believes that the reasonable implementation of PrEP and PEP is currently the fundamental measure to prevent human deaths from rabies. After the rabies virus invades the body through animal bites or scratches, it usually stays at the site of entry for several weeks or months. After exposure to the rabies virus, the wound should be thoroughly washed with running water or alkaline soapy water immediately and promptly treated at a rabies clinic in a standardized manner. Modern rabies vaccines should be administered in full on time. For those with grade III exposure or exposure sites close to the central nervous system, rabies passive immunization preparations should be injected promptly. The initial full immunization of human rabies vaccine requires 14 doses, and there is also a 23-dose method. For high-titer cell culture vaccines, Europe was the first to adopt the “6-dose method”, which means a vaccination schedule of six consecutive injections on the 0th, 3rd, 7th, 14th, 28th and 90th days. A large amount of clinical data indicates that the sixth dose of vaccination does not significantly increase the antibody level in the recipient’s body. Currently, the “five-dose method” (Essen method) is widely adopted worldwide. The new immunization schedule has been further reduced to four doses, namely the “2-1-1” schedule (Zagreb method) and the “simple four-dose method”.
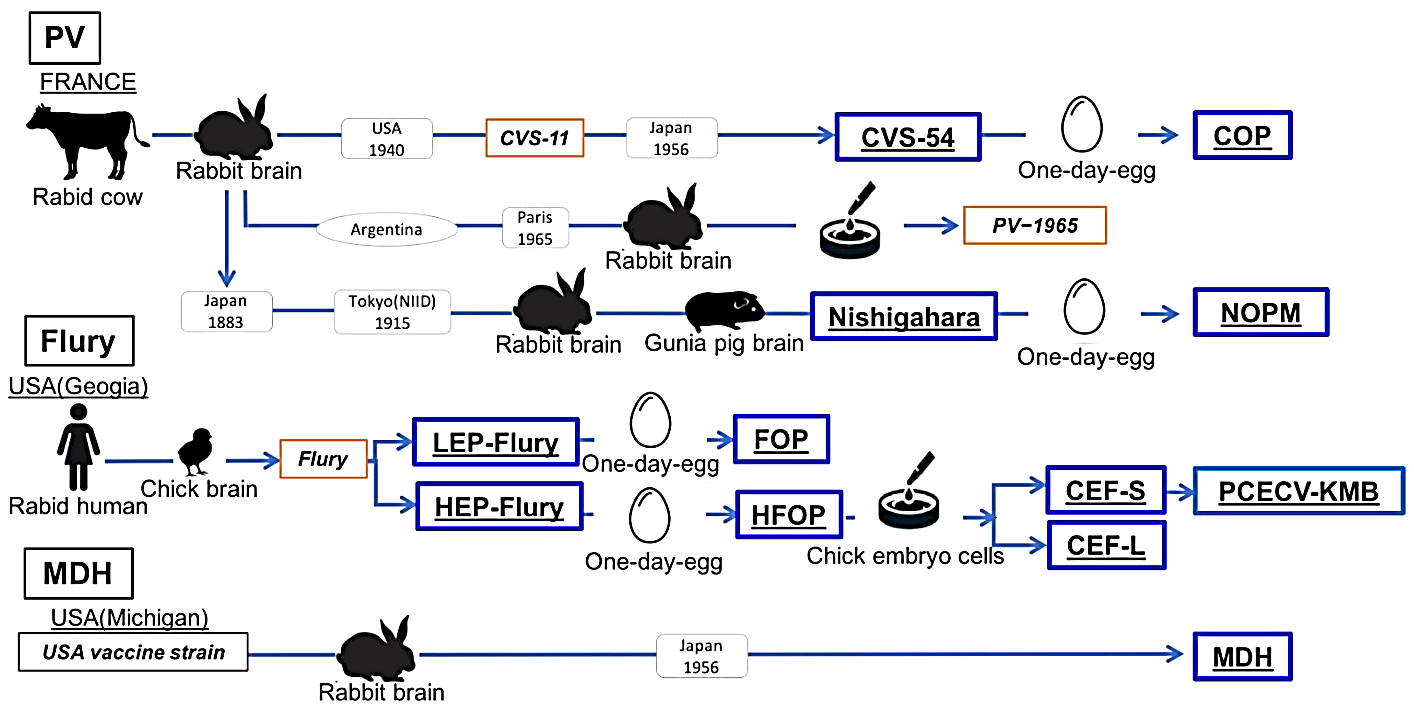
Figure 1: Schematic diagram of the fixed strain lineage used as vaccine seeds [3]
New immunization techniques, such as oral rabies vaccines and biodegradable “biological needles” based on silicon or starch polymers, are regarded as ideal ways to reduce production costs and minimize the waste of consumables. Fundamental research has been conducted on the application of reverse genetics technology in the development of new rabies vaccines. Significant achievements have also been made in the development of substitutes for human rabies vaccines such as plant-derived antibodies and human antibodies (Figure 1). The recombinant human rabies virus monoclonal antibody (recombinant human rabies immunoglobulin, rhRIG) developed by a domestic enterprise has undergone phase II clinical trials, where shows a good result [2]. Here, this research will discuss common rabies treatment methods and analyze their specific therapeutic effects.
2. Treatment methods analysis
2.1. Antiviral drugs
In the continuous exploration, people have discovered potential anti-RABV drugs and targets. Antiviral drugs can not only be used for treatment, but also have the advantages of easy preservation, easy production and moderate cost. Therapeutic antibodies for rabies are costly, difficult to preserve and have complex review procedures. Therefore, for RABV, it is easier to find anti-RNA virus drugs than vaccines and therapeutic antibodies. The main drugs known to have inhibitory activity against RABV are ribavirin, amantadine and interferon IFN-α. Ribavirin is a purine analogue and RNA mutagenic agent that can be incorporated into the templates of cytosine nucleotides and uracil nucleotides with the same efficiency to induce mutations. Studies have shown that ribavirin has in vitro activity against RABV infection. However, after intracerebral injection of ribavirin, it was found that it had no efficacy against mice infected with the RABV street strain. Due to the lack of therapeutic effect and adverse reactions of ribavirin in the treatment of rabies patients, more research and discussion are still needed on whether ribavirin can treat rabies. Interferons, especially type I interferons (IFN-α/β), are important defense measures against viral infections, including Toll-like receptors (TLR) and cytoplasmic retinoic acid-induced gene I. After RIG-I recognizes Pathogen-associated molecular patterns (PAMP), they are produced in almost all cells and induce the activation of a cascade reaction to produce type I interferons. After RABV enters the nervous system, it triggers innate immune responses, including type I interferon responses. This response limits the transmission of RABV in the muscles at the vaccination site and slows its metastasis to the spinal cord, thereby delaying the death of mice.
At present, experiments have proved that treatment with exogenous interferon can prevent RABV from replicating in cell cultures, and treatment can be carried out before or after infection [4]. Treatment with exogenous interferon or interferon inducer (Poly I:C) can partially or completely inhibit RABV infection in mouse and rhesus monkey models. The therapeutic effect is higher when this compound is used in large doses before and after virus infection and as close as possible to the virus inoculation site. Amantadine is a synthetic antiviral drug and also a weak non-competitive N-methyl-D-aspartic acid receptor (NMDA) antagonist, which can inhibit the replication of the virus in cells. Two in vitro studies have evaluated the role of amantadine in RABV infection. One of them is that amantadine can interfere with the replication of RABV stationary strain (CVS-11) in CER cells, and its dose-dependent inhibitory effect ranges from 10% inhibition at 25 μg/mL to 99.9% inhibition at 250 μg/mL. When the drug is pre-incubated with the cell and removed before viral infection, or when amantadine is added to the virus during the adsorption process, amantadine does not inhibit viral replication. This indicates that the attachment and penetration of the virus may not be affected by the drug, and therefore amantadine may hinder the unpacking of the virus [5].
2.2. RNA vaccines
RNA vaccines are a new type of vaccine that has emerged in recent years and represent a new generation of nucleic acid vaccines. RNA vaccines mainly include self-amplifying RNA (self-amplifying RNA, saRNA) vaccines, mRNA vaccines and circularRNA (circRNA) vaccines. The saRNA vaccine can self-replicate after entering cells, and a very small amount can produce a large amount of antigen proteins. mRNA vaccines are currently the most maturely studied and have the most perfect preparation processes. The circRNA vaccine is the most stable among the three vaccines because it can form a ring by itself and has a stable structure. There are mainly four types of delivery system platforms for nucleic acid vaccines: polymer-based carrier platforms, polypeptides such as protamine as carrier platforms, pure nanoparticles (NPs) as carrier platforms, and LNP as carrier platforms. The most widely used mRNA vaccine vector platform is LNP. LNP has the following advantages. It is not degraded by enzymes and can be efficiently delivered to the cytoplasm with prolonged stability. LNP can also enhance the long circulation time of mRNA, possess high fusion ability with cell membranes and endosomal escape ability. To sum up, the advantages of RNA vaccines include: flexible design, strong immunogenicity, low risk of gene recombination, short research and development cycle, and good safety. In the research system of RNA vaccines both at home and abroad, only the research on mRNA vaccines is relatively mature. Moreover, the inactivated vaccines currently in use are immunized after exposure, and the 4-dose or 5-dose method is adopted. The large number of immunizations leads to a complicated immunization schedule. mRNA is expected to reduce the number of vaccine immunizations, optimize the immunization schedule, and facilitate post-exposure immunization. For animals, only one dose of mRNA vaccine is needed to reach the immune level, saving a lot of labor costs. The research on RNA vaccines plays a crucial role in eliminating the transmission of rabies and increasing the vaccination rate of rabies among animals in the future [6].
2.3. Research on rabies vaccine for humans
The development of new rabies virus vaccine adjuvants that are efficient, low-toxic, safe and inexpensive has become an important field in the current research of rabies vaccines [7]. Pica adjuvant is a TLR3 agonist that can activate effective antigen-presenting cells, such as dendritic cells, thereby generating a more powerful adaptive immunity. Compared with the standard immunization schedule of commercially available vaccines, the accelerated immunization schedule of picazadjuvant rabies vaccine has good tolerance in healthy adults and shows non-adverse immunogenicity. On the 7th day after vaccination, the neutralizing antibody seroconversion rate of the rabies vaccine recipients with picazol was 57.6%, and the GMT level (0.60 IU/mL) were both higher than those of the control group, which were 43.8% and 0.39 IU/mL. The test results on the 14th day after vaccination were similar. Pickup adjuvant achieves RVNA≥ 0.5 IU/mL more quickly. The study did not identify any new or clinically significant safety issues. CV8102 is a TLR 7/8/RIG I agonist RNA adjuvant. The first human trial study showed that when CV8102 was administered alone or in combination with rabies vaccine, it mainly caused grade 1 or 2 local or systemic reactions, and no related serious adverse reactions occurred. The combined vaccination of 25-50 mg CV8102 with rabies vaccine can significantly enhance the immunogenicity of rabies vaccine. The results of a Phase I clinical trial of an mRNA vaccine based on protamine (CV7201) in healthy adults has been developed. The results of this first clinical study conducted in a healthy population show that CV7201 can induce functional antibodies against viral antigens when immunized with a needle-free device, but it cannot produce an effective immune response when immunized with a syringe with a needle. The vaccine is generally safe and has reasonable tolerance. However, in view of the safety issues of CV7201, researchers further developed the mRNA rabies vaccine CV7202 encapsulated with lipid nanoparticles. The results of the Phase I clinical study showed that the doses of 1 μg and 2 μg of CV7202 were well tolerated, and there were no serious adverse events related to the vaccine.
The research on the rabies candidate vaccine based on monkey adenovirus vector has shown good safety and immunogenicity. ChAdO×2RabG is a rabies candidate vaccine using monkey adenovirus as a vector. A first-in-human study is aimed to evaluate its safety and immunogenicity in healthy adults. Twelve healthy adults were divided into groups receiving low-dose, medium-dose and high-dose ChAdO×2RabG3. The subjects did not report any serious adverse events. ChAdO×2RabG demonstrated acceptable safety and tolerability, as well as encouraging immunogenicity. Research on the novel recombinant nanoparticle rabies virus glycoprotein vaccine has shown good safety and immunogenicity. A new type of recombinant nanoparticle rabies virus vaccine can be developed. In the Phase III clinical study, the results showed that approximately 9.9% of the subjects in the recombinant rabies virus glycowhite vaccine group and approximately 17.2% of the subjects in the control group reported adverse events. It can be seen that the new three-dose recombinant rabies virus glycoprotein vaccine for simulated post-exposure prophylaxis is not inferior to the five-dose vaccine that has passed the prequalification of the WHO in terms of safety and immunogenicity. Researchers believe that the reduction in the number of vaccine doses leads to a decrease in the number of visits and transportation costs, and increases compliance, which is very important for the prevention of rabies [8].
3. Conclusion
Anti-rabies virus drugs can not only be used for treatment, but also have the advantages of easy preservation, easy production and moderate cost. It is simpler to find anti-RNA virus drugs for RABV than vaccines and therapeutic antibodies. The main drugs known to have inhibitory activity against RABV at present are ribavirin, amantadine and interferon IFN-α. After intracerebral injection of ribavirin, it was found that it had no efficacy against mice infected with the RABV strain. Due to the lack of therapeutic effect and adverse reactions of ribavirin in the treatment of rabies patients, more research and discussion are still needed on whether ribavirin can treat rabies. The advantages of RNA vaccines are good safety, high immunogenicity, flexible design, and short research and development cycle. Among the research systems for RNA vaccines both at home and abroad, only the research on mRNA vaccines is relatively mature. mRNA is expected to reduce the number of vaccine immunizations. Pica adjuvant is a TLR3 agonist that can activate effective antigen-presenting cells. The accelerated immunization program of Pica adjuvant rabies vaccine has good tolerance in healthy adults and shows non-adverse immunogenicity. SCV8102 is a TLR 7/8/RIG I agonist RNA adjuvant. When administered alone or in combination with rabies vaccine, no serious adverse reaction events occurred. The mRNA rabies vaccine CV7202 encapsulated with lipid nanoparticles can be used. The results of the Phase I clinical study showed that the doses of CV7202 at 1 μg and 2 μg were well tolerated, and there were no serious adverse events related to the vaccine. The research on the rabies candidate vaccine based on monkey adenovirus vector has shown good safety and immunogenicity. ChAdO×2RabG is a rabies candidate vaccine using monkey adenovirus as a vector. Acceptable safety and tolerability were demonstrated in the research. The novel recombinant nanoparticle rabies virus glycoprotein vaccine has shown good safety and immunogenicity. Enhancing people’s awareness of the importance of vaccination to prevent rabies, increasing the coverage rate of preventive vaccination among humans, and strengthening canine vaccination activities have also played an important role in eliminating rabies.



