1. Introduction
1.1. Chemical and physical property
The molecule formula for menthol is C10H19OH, and the skeletal formula is shown in figure 1. As a kind of alcohol, menthol has a general property of alcohol such as esterification and oxidation. Due to the existence of methyl, isopropyl and oxhydryl (-OH), the menthol molecule has 3 optical centres, which means there are actually 8 stereoisomers of menthol. They all have the same chemical properties but they have different physical properties such as boiling points. There is one thing to point out is that this paper will only talk about the synthesis process of l-menthol, which is also called as (-)-menthol hereinafter. To only produce l-menthol chemists have been working on it for many years, there are 3 distinct routes in total.
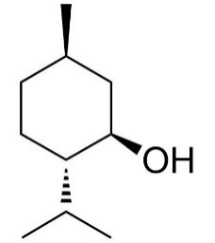
Figure 1. l-menthol [1].
1.2. The importance of menthol
As the abstract previously mentioned, menthol is widely used in the manufacture of many products, notably, menthol has the functions of easing pain and apocatastasis in pharmacy, hence menthol is also widely used in many medicines. Menthol is an important aroma chemical that occurs naturally in peppermint oil and is widely used as a cooling agent [1]. Naturally sourced peppermint oil can vary in both the relative composition of its monoterpenoid constituents and overall yields due to fluctuating environmental conditions. This leads to price volatility and increased arable land competition for more profitable crops, such as biofuel biomass production [2]. In conclusion, the researches on menthol synthesis are very important.
2. The Takasago synthesis
2.1. Introduction
Nowadays the Takasago synthesis (Figure 2) is the most widely used synthesis route of manufacturing menthol. Takasago International Corporation produces more than 2000000 kg of L-menthol per year [1]. The kay step of the entire reaction is the second step –adding 2,2‘-bis(diphenylphosphino)-1,1‘-binaphthyl (BINAP)-Rh complex, which is an enantioselective catalyst. This reaction will mostly form one stereoisomer, and proceeds in 96–99% ee [1]. The ee number shows the ratio of the target enantiomer to the other enantiomers. The closer to one the value is, the more the target product will be produced. Hence, the reaction with a higher ee is usually seen as a more excellent reaction. The concept of ee will be used massively in the rest of this paper, and readers should be familiar with it. It should be pointed out that the main logic of these 3 routes is to form the (R)-Citronellal. The rest of the steps are totally the same. The detailed mechanism is shown in Figure 3.
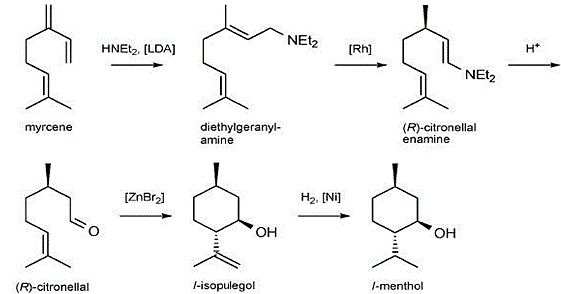
Figure 2. Takasago l-menthol process [1].
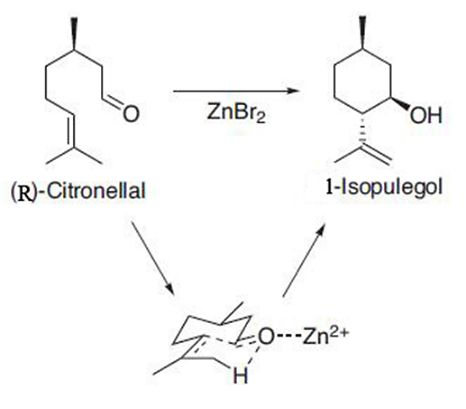
Figure 3. detailed description of the addition of zinc bromide [3].
2.2. Advantages and disadvantages
At present, the Takasago synthesis is quite mature and fully developed. Despite Takasago International Corporation itself, many other factories also manufacture menthol through this pathway because this route has large output. Regardless of which of these 3 methods is used, natural products are essentially required. The synthesis of L-menthol mostly requires natural products as raw materials, which are relatively expensive [4]. Hence, lowering production costs in the synthesis process has already become a strong pursuit of various chemical companies which are engaging in menthol production. The advantage of having large output is the most competitive and persuasive point in factories owner′s eyes. That is why the Takasago synthesis route still has a very high market share. However, the disadvantages are significant, too. There are 5 steps in total, which will cause a higher cost of the reagents and more energy consumption. Besides, the initial material –myrcene is also more expensive than the initial material for the rest two routes(E/Z mixture of citral). It is also hard to extract the target product from the final mixture. But the ee for this process is pretty high (about 96% to 99%), hence the Takasago synthesis is used extensively nowadays.
3. The BASF synthesis
3.1. Introduction
On the Takasago process′s basis, the BASF basically simplified the entire route and replace the initial reagent to citral which is easier and cheaper to purchase. The biggest change in the 1st step is rectification which is a purely physical process. The rectification is normally carried out in a distillation tower, where the gas-liquid two phases conduct interphase heat and mass transfer through counter-current contact. The volatile components in the liquid phase enter the gas phase, while the non-volatile components in the gas phase enter the liquid phase. As a result, almost pure volatile components can be obtained at the top of the tower, and almost pure non-volatile components can be obtained at the bottom of the tower [5]. The rectification is a useful way to extract the target optical isomer by using their different boiling points. This is a simple but effective step to form a wanted enantiomer. In many industrial processes, rectification is a rather common step. The figure 4 shows the whole BASF synthesis.
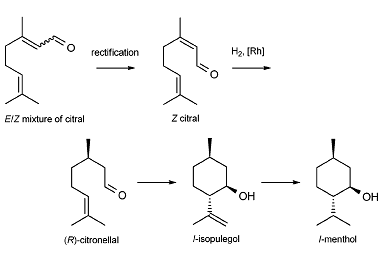
Figure 4. BASF l-menthol synthesis [1].
3.2. Advantages and disadvantages
One significant improvement is that the citral is much easier to produce, and the yield will also increase. While the citral produced by BASF is fossil-based, its synthesis has the potential of including renewable feedstocks in the future, making its menthol synthesis more attractive from a sustainability viewpoint [3]. However, the rectification of E/Z mixtures to obtain high-purity Z-citral (neral) is needed in their method [1]. the other disadvantage is the BASF synthesis does not avoid using the high-priced rhodium catalyst. These two main disadvantages are very considerable for the whole route, and that is why many companies around the world are still using the Takasago synthesis rather than BASF.
4. The new route and its burgeoning dual catalyst system
4.1. The detailed explanation of the dual catalyst system
As its name indicates, this system has two parts of catalysts which are heterogeneous Pd/BaSO4 catalyst, and a tiny load of chiral cyclic amine was then used, which acts as an asymmetric organocatalyst. The use of the dual catalyst system massively increases the rate of the whole reaction, furthermore, the proper sort of amine is able to influence the yield and conversion noticeably. Chemists have successfully worked out the data for some particular amines, and it can be looked up in Table 1 (the experiment was carried out in the presence of 5%-Pd/BaSO4 (0.1 mol%), chiral amines (1.6–1.9 mol%), and trifluoroacetic acid (TFA) (1.6–1.9 mol%) under atmospheric pressure of H2 in t-BuOH/H2O (92: 8 v/v) at 50 ℃ the BuOH is normally called as butane-1-ol and the initial ratio of E-citral and Z-citral is 50:50.) The inner essence of the amine catalyst working principle is rather complex, the full 3D rection process of how the amine catalyst works is shown in Figure 5, the first step of the reaction is activation with the existence of proton and water. The substance 6 in Table 1 plays a role as acid and 6′works as a conjugate base. The para position of benzene increases the PKA value of 6 which means it will be more acidic hence the equilibrium will shift to the right. The whole reaction is more likely to happen. That is why the para position will increase the ee. In conclusion, the new and efficient dual system is a revolutionary discovery in modifying the synthesis route of menthol. With the deepening of research, the most suitable amine will absolutely be found. The corresponding research is still approaching This system is able to increase the yield of the final product, and its enantioselectivity is also quite remarkable in the research stage. With the promotion of this system, maybe the E/Z mixture of citral can have an arbitrary ratio one day. The research in this domain is very promising.
Table 1. Scope of amine catalyst for asymmetric hydrogenation of citral [1].
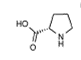
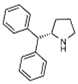
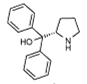
(S)-Proline |
(S)-1a |
2 |
3 |
4 |
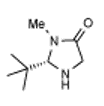
Entry | Amine | Conversionb(%) | Yieldb(%) | %eec |
1d | (S)-Proline | 100 | 37 | 3(S) |
2 | (S)-Proline | 52 | 38 | 6(S) |
3 | (S)-1a | 75 | 62 | 77(S) |
4 | 2 | 82 | 55 | 40(S) |
5d | 3 | 85 | 35 | 5(S) |
6 | 3 | 52 | 39 | 69(S) |
7 | 4 | 96 | 72 | 74(S) |
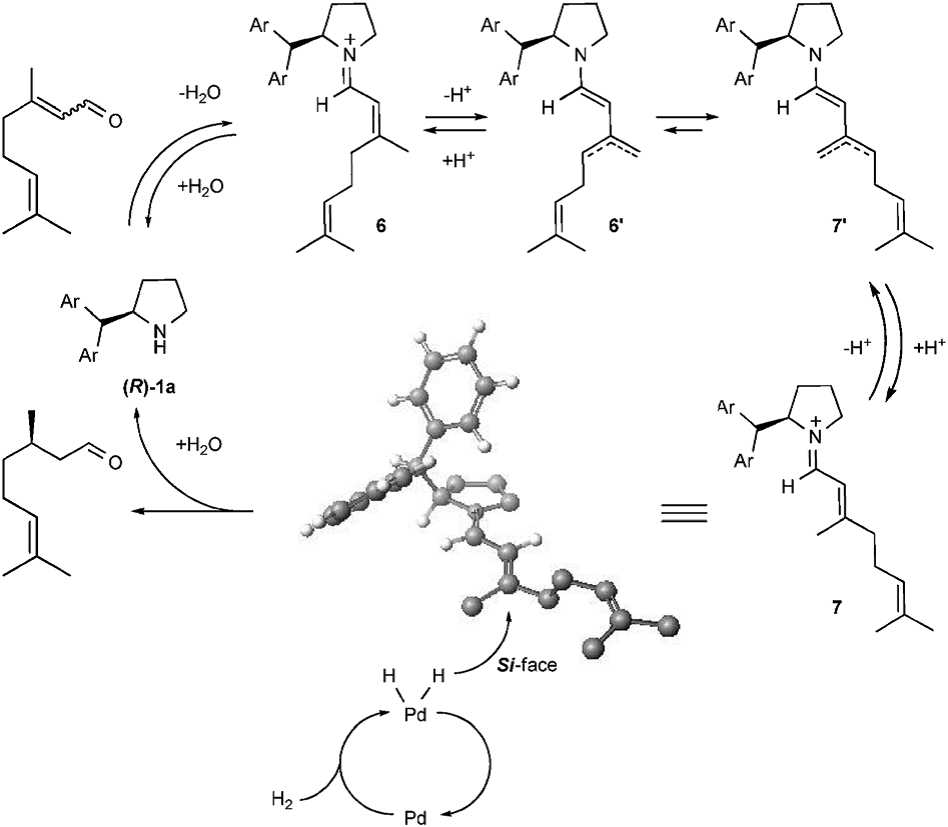
Figure 5. Plausible reaction mechanism [1].
4.2. Simple introduction of the new synthesis route using the dual catalyst system
Generally, the new route is polished up from the foundation of BASF route. Under the influence of dual catalyst system, the first two steps are merged into one step. The use of a dual catalyst system has significantly modified the whole reaction route. The new route is shown in Figure 6.
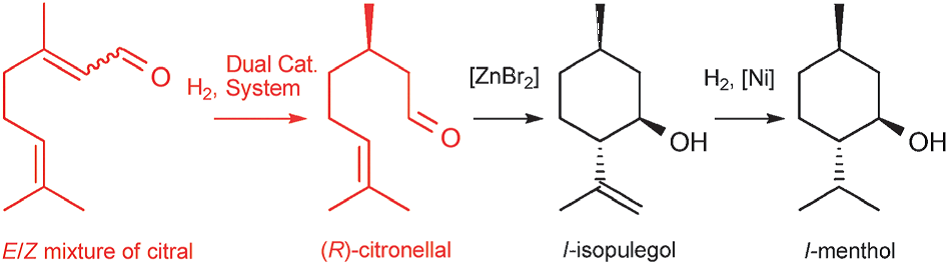
Figure 6. New route to L-menthol [1].
4.3. Advantages and disadvantages
The first advantage is the dual catalyst system significantly enlarged the rate of the reaction, and the energy-consuming rectification step is abandoned. The research of the dual catalyst system is still being underway, so the ee value for the reaction is a little bit lower than the Takasago process, the highest value is about 88% or 89%, but as this work previously talked about, this research is still going, the chemists will try their best to achieve higher ee and conversion in the future. The ee for Takasago synthesis is too demanding for the new route now. For today, the low ee value is still a non-negligible disadvantage. The advantages are very clear, the whole reaction is further simplified, and the dual catalyst is easy to be recycled. Another benefit is the dual catalyst is, much cheaper than the rhodium catalyst. Despite with low ee, another disadvantage is it does not change the remaining steps of adding zinc bromide and hydrogen with Ni catalyst. It will make the final mixture still not easy to extract the menthol molecule itself. As a whole, the advantages are much more significant than the disadvantages.
5. Conclusion
After comparing with the other two synthesis routes, the Takasago process is still having more economic advantages. Most of companies still choose this route to make menthol. Almost every rest of the companies use the BASF synthesis, because it is more environmentally friendly and the citral is cheaper. The new route is still in the experimental stage and it has not been put into practise at present, but many enterprises have already put a large amount of funds in developing the dual catalyst system. Looking ahead of this domain, the development of the dual catalyst system is the most promising research without a doubt. As soon as the proper amine is found, the reality of putting this revolutionary discovery into practise won′t be very far. By then, the production of menthol will definitely be advanced. Even though the Takasago synthesis maybe will still possess more market share in the next dozens of years, the new route is still going to a very bright future, and I believe that one day it will definitely replace the Takasago process.