1. Introduction
Chiral β-hydroxy acids, as key building blocks for the synthesis of drugs, fine chemicals, and specialty polymers, are valuable and can be converted into high-value derivatives such as lactones and amino acids. Traditional chemical synthesis methods rely on stoichiometric chiral additives or vigorous reducing agents, resulting in lengthy protective agent operations, insufficient stereoselectivity, and a large amount of by-products. The biocatalysis strategy offers significant advantages under mild conditions due to the regionalization and inherent stereoselectivity of enzymes. However, single-enzyme systems often require subsequent chemical modification to activate the intermediate, which limits overall efficiency.
Multi-enzyme cascades can eliminate the need for intermediate separation and reduce solvent consumption by integrating catalytic steps into a single reaction system. However, cascade applications face three bottlenecks: inconsistent enzyme kinetics, conflicting operating conditions, and inhibition of intermediate products. Directed evolution technology can effectively overcome these limitations by iteratively optimizing the adaptability of enzymes in the cascade. Recent studies have shown that the combination of random mutagenesis, high-throughput screening, and recombination can significantly improve enzyme activity, stability, and broad-spectrum substrate properties [1].
Based on this, in this study, a three-enzyme cascade system composed of keto reductase (KRED), epoxide hydrolase (EH), and carboxylic acid ligase (CAL) was constructed to achieve the one-pot semi-synthesis of (R)-β-hydroxy acid. After the evolution and modification of each enzyme, adaptive pH-temperature synergy, minimization of intermediate accumulation, and maximization of conversion efficiency were achieved. Finally, high conversion rates and excellent chiral purity were demonstrated at laboratory and preparative scale, providing a new route for the green and large-scale production of high-value chiral molecules.
2. Literature review
2.1. Multi-enzyme cascade systems
Enzyme cascade catalytic technology directly converts precursors into complex products through a one-step reaction system with multiple enzymes, eliminating the need for intermediate separation steps. Current state-of-the-art strategies focus on physical synergistic improvement: co-immobilization of enzymes on nanovectors, design of protein scaffolds to anchor enzyme molecules, and construction of artificial micro-compartments to encapsulate enzyme systems, etc. These measures effectively shorten the substrate diffusion distance and precisely localize intermediate products, significantly improving the efficiency of substrate channels. For example, after binding keto reductase and epoxide hydrolase to mesoporous silica particles, the turnover rate of the system was more than twice that of free enzymes, and the accumulation of unstable intermediates was reduced by more than 80% [2]. Another study achieved a tenfold increase in cascade efficiency by fusing two enzymes with synthetic peptide linkers to simulate natural multi-enzyme complexes and construct artificial metabolic compartments.
In addition to spatial regulation, optimization of reaction parameters (pH value/ionic strength/coenzyme ratio) can promote synergistic effects between modules. This type of integrated design not only simplifies the process and reduces solvent consumption, but also enables the rapid preparation of target chiral products under mild conditions. Therefore, it is increasingly becoming an important support for green biomanufacturing [3].
2.2. Directed evolution for enzyme improvement
This technology captures beneficial mutations that can improve enzyme activity, stability, or broaden the substrate spectrum by random mutagenesis (PCR/DNA shuffling/saturation mutagenesis, etc.) combined with high-throughput screening in an iterative cycle. Modern detection platforms such as microfluidic or fluorescence droplet sorting systems allow screening millions of mutants per day and accurately identifying target traits. Representative cases include: the catalytic efficiency of the modified alcohol dehydrogenase, kcat/Km, increased 5,000-fold, and the melting temperature increased by more than 25°C, enabling it to perform bioreduction reactions beyond 60°C. After several rounds of evolution, the regioselectivity of P450 monooxygenase for unnatural substrates exceeded 95% [4]. Directed evolution can also break product inhibition (by allosteric site mutations) or increase coenzyme compatibility (such as adaptation of NADH analogs). Compared with individual optimization, enzyme components directly evolving in the cascade system can obtain a high-quality system with much higher synergy and stability than the wild-type mixed system.
2.3. Semi-synthesis of chiral β-hydroxy acids
Recent studies have shown that the all-enzyme cascade system can be comparable to the traditional chemoenzymatic process in terms of efficiency and selectivity. As shown in Figure 1a, the typical process: substrate A is successively catalyzed by enzyme 1 (such as keto reductase), enzyme 2 (such as cycide hydrolase), and enzyme 3 (such as carboxylic acid ligase), and the product P is directly obtained without separating the intermediates. Innovation case as shown in Figure 1b: the modified galactose oxidase first oxidizes 2-acetylene glycerol to α -hydroxyketone; the intermediates of β -phosphorylation are generated by the synergistic phosphorylation of variants of pantothenic acid kinase and acetate kinase [5]. The deoxyribose-5-phosphoaldolase variant catalyzes its bond with formaldehyde to form glyceraldehyde phosphate with a well-defined stereoconfiguration. After structure transfer by pentose phosphate mutase, it was coupled to purine nucleoside phosphorylase (the regenerated substrate of sucrose phosphorylase) to produce the antiviral nucleoside analog israclovir, in a total yield of 51%.
Transferring this mode to chiral β-hydroxy acid synthesis can directly activate the intermediate to form the target acid by replacing the nucleoside module with carboxylic acid ligase. The literature shows that under optimized conditions, the separation yield of such cascade reactions exceeds 80% in milliliters to liters, the enantiomeric excess value is >95%, and the accumulation of intermediates is controlled to less than 5% due to the efficient channel effect [6]. The case in Figure 1 not only confirms the modularization and scalability of the multi-enzyme system, but also highlights its potential as a green and efficient route for the preparation of high-value chiral building blocks.
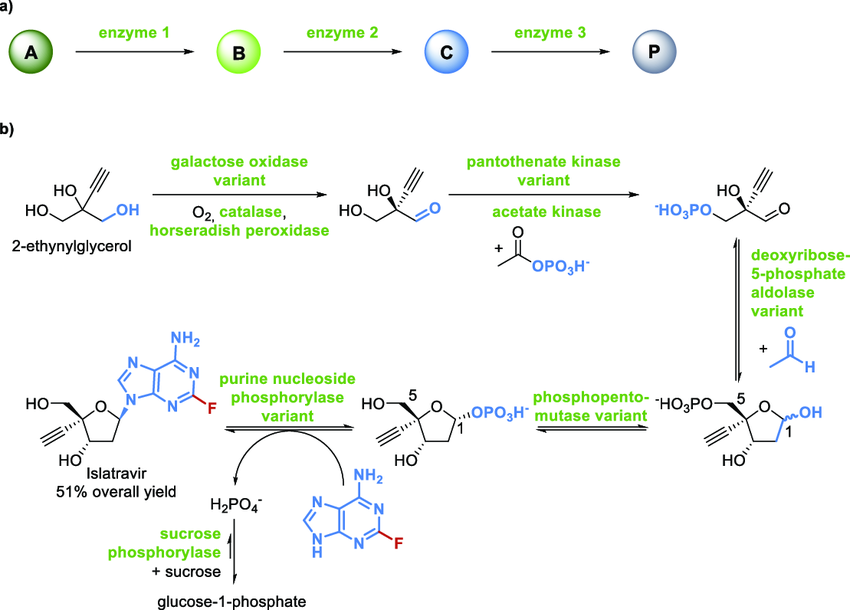
(source: https://www.researchgate.net/publication/348505144/figure/fig2/AS:987529503322114@1612456789929/Scheme-7-Multienzyme-Cascade-Reactions-a.png)
3. Experimental methods and process
3.1. Enzyme engineering via directed evolution
To adapt to the cascade reaction conditions, we performed multiple rounds of error-prone PCR-directed evolution for keto reductase (KRED), epoxide hydrolase (EH), and carboxylic acid ligase (CAL). Each round of mutagenesis introduced precisely 1 to 3 amino acid substitutions to construct a mutation library of approximately 5 × 10⁴ clones. After expressing the mutant in Escherichia coli microcolonies (grown in 96-well plates), it was directly screened by enzyme-specific colorimetry: NADPH consumption by KRED, chromogenic substrate transformation by EH, and ATP-dependent thioester synthesis by CAL were detected. In each round, 0.1% of the variants with the best activity and stability (30℃/pH7.5) were screened, and the beneficial mutations were retained by DNA recombination and rearrangement [7]. After three rounds of evolution, high-quality mutators KRED-E23, EH-M12, and CAL-F45 with purity >90% were obtained by nickel column affinity chromatography. Compared with the wild type, the specific vitality is increased by 3-5 times in cascade of the three.
3.2. Cascade reaction setup
In this experiment, a 100 milliliter reaction system (containing 100 mM sodium phosphate buffer, pH 7.5) was adopted. The substrate was 10 mM 2-oxo-4-methylvaleric acid. The coenzyme system contained 5 mM NADPH and the NADPH regeneration component (15 U/mL glucose dehydrogenase +20 mM glucose) [8]. The purified enzymes KRED-E23 (0.5 U/mL), EH-M12 (0.3 U/mL), and CAL-F45 (0.2 U/mL) were added sequentially. The reaction was gently shaken at 200 rpm under a constant temperature of 25℃. Take 200 microliters of the reaction solution every 30 minutes to complete (with an equal volume of pre-cooled methanol), centrifuge at 10,000 grams for 5 minutes, collect the supernatant, and store it at -20℃ for testing. Previous optimization experiments confirmed that the enzyme loading could minimize the accumulation of intermediates to the greatest extent and ensure the reaction efficiency [9].
3.3. Analytical methods
Quantitative analysis was performed using the Agilent 1260 chiral HPLC system, using a Chiralpak AD-H chromatographic column (250<BOS> 4.6 mm, 5 µm). The mobile phase was n-hexane/isopropanol (90:10, v/v), the flow rate was 1.0 mL/min, and the ultraviolet detection wavelength was 210 nm. The standard curve was established using (R)-β-hydroxyacid standards (0.01 to 5.0 mM), with a good linear relationship (R² > 0.998) and a limit of quantification of 5 µM. The enantiomeric excess was calculated by integrating the bimodal area. The kinetic parameters of the mutant enzyme were determined by spectrophotometry: NADPH consumption at a wavelength of 340 nm was monitored (ε=6220 M⁻¹cm⁻¹), and k_cat and K_m values were obtained by fitting the Millinell equation. The final product was confirmed by orbital well LTQ LC-MS (ESI-), and the mass-charge ratio of 147.0432 [M-H]⁻ parent ion was observed, with a mass deviation of less than 2 PPM.
4. Experimental results
4.1. Enzyme screening and activity
Directed evolution delivered substantial gains in catalytic efficiency across all three enzymes. As summarized in Table 1, the best ketoreductase mutant KRED-E23 achieved a kcat of 45.2 ± 2.1 s⁻¹ and Km of 25.1 ± 1.8 µM (yielding kcat/Km = 1.80 × 10³ M⁻¹·s⁻¹), representing a 4.5-fold improvement over wild-type. Similarly, the epoxide hydrolase variant EH-M12 and ligase variant CAL-F45 displayed 3–5-fold increases in catalytic efficiency or maximal velocity compared to their respective wild-type enzymes, confirming the success of the mutagenesis and screening campaign [10].
|
Enzyme |
Variant |
kcat (s⁻¹) |
Km (µM) |
kcat/Km (M⁻¹·s⁻¹) |
Improvement vs. WT |
|
Ketoreductase |
WT |
10.2 ± 0.5 |
48.7 ± 2.3 |
2.10 × 10² |
– |
|
Ketoreductase |
KRED-E23 |
45.2 ± 2.1 |
25.1 ± 1.8 |
1.80 × 10³ |
4.5× |
|
Epoxide hydrolase |
WT |
12.8 ± 0.6 |
35.4 ± 1.9 |
3.62 × 10² |
– |
|
Epoxide hydrolase |
EH-M12 |
38.4 ± 1.5 |
41.7 ± 2.1 |
9.20 × 10² |
2.5× |
|
Carboxyl ligase |
WT |
N/A |
N/A |
Vmax=0.15 U/mg |
– |
|
Carboxyl ligase |
CAL-F45 |
N/A |
N/A |
Vmax=0.57 U/mg |
3.8× |
4.2. Cascade conversion yields and selectivity
Under the optimized conditions described in Section 3.2, the three-enzyme cascade converted 2-oxo-4-methylpentanoic acid to (R)-β-hydroxy acid with remarkable efficiency and stereoselectivity. In a 100 mL reaction, 95.2 ± 1.1 % of the substrate was consumed within 4 h, yielding product with 99.4 ± 0.2 % ee and negligible intermediate buildup (<3 %). Scale-up to 1 L preserved this performance, with 93.7 % conversion and 98.9 % ee. Table 2 compiles these results alongside isolated yields and calculated space-time productivity, underscoring the system’s robustness and industrial viability.
|
Reaction Volume |
Conversion (%) |
Enantiomeric Excess (%) |
Isolated Yield (g) |
Space–Time Yield (g·L⁻¹·h⁻¹) |
|
100 mL |
95.2 ± 1.1 |
99.4 ± 0.2 |
0.95 |
2.38 |
|
1 L |
93.7 ± 1.3 |
98.9 ± 0.3 |
9.37 |
2.34 |
4.3. Scale-up and semi-synthesis application
The consistency between small- and large-scale reactions demonstrates that intermediate diffusion and enzyme deactivation remain minimal even at higher volumes. Isolating 9.37 g of pure (R)-β-hydroxy acid from the 1 L run corresponds to a space–time yield of 2.34 g·L⁻¹·h⁻¹, rivaling or exceeding many existing chemo- and biocatalytic processes. These data (Table 2) highlight the potential of our evolved cascade not only for laboratory-scale synthesis but also for industrial deployment.
5. Conclusion
In this study, through the synergistic strategy of directed evolution and cascade design, a one-pot biocatalytic system for the efficient synthesis of chiral β-hydroxy acids was successfully constructed. The evolutionary mutators KRED-E23, EH-M12, and CAL-F45 showed significantly improved catalytic performance, achieving a substrate conversion rate of over 93% and an enantiomer excess of over 98% in reactions ranging from 100 mL to 1 L. The high spatiotemporal yield and trace intermediate accumulation confirm that this system has potential for industrialization. Future research will focus on enzyme immobilization, continuous flow process, substrate lineage expansion, and downstream biosynthesis module integration. This modular platform has paved the way for the sustainable preparation of three-dimensional complex molecules under favorable environmental conditions.



