1. Introduction
Lovastatin, a member of the statin class of drugs, plays a critical role in lowering lipid levels, particularly low-density lipoprotein cholesterol (LDL-C), and is widely used to prevent cardiovascular diseases [1]. It was first isolated as a natural product from the fungus Aspergillus terreus, discovered by Alberts and colleagues in the late 1970s [2]. Lovastatin belongs to the group of HMG-CoA reductase inhibitors, which competitively inhibit the enzyme HMG-CoA reductase, preventing the conversion of HMG-CoA to mevalonate, a critical step in the biosynthesis of cholesterol [3,4]. By reducing cholesterol levels, especially LDL-C, lovastatin significantly decreases the incidence of atherosclerotic cardiovascular diseases, particularly in individuals with hypercholesterolemia [5].
In addition to its lipid-lowering effects, lovastatin has been shown to exhibit several other biological activities. Increasing evidence suggests that statins may possess anti-inflammatory, antioxidant, and even anti-cancer properties [6]. Studies indicate that lovastatin may exert antitumor effects by inhibiting the Ras signalling pathway [7]. Although these non-conventional uses are still under investigation, they provide new avenues for the application of statins beyond cardiovascular diseases [8].
However, despite the significant benefits of lovastatin and other statins, long-term use of these drugs can lead to several side effects. The most common are muscle-related side effects, such as myalgia and myopathy, with rare cases of severe rhabdomyolysis [9]. Additionally, lovastatin use has been associated with elevated liver enzymes, necessitating regular monitoring of liver function [10]. Therefore, while lovastatin remains a frontline treatment for hypercholesterolemia, proper dosage management and monitoring are critical.
The development of lovastatin has also sparked significant interest in its metabolic and biosynthetic pathways. In particular, research into microbial biosynthesis of lovastatin and its metabolites has provided valuable insights for developing more efficient and selective statin drugs [11]. The application of gene editing technologies to regulate lovastatin’s biosynthetic pathways has become an active area of research in recent years [12].
The synthesis of compounds 1-3, novel lovastatin derivatives characterized by a tetracyclic ring system, is of significant interest due to their potential biological activities and structural uniqueness.
Figure 1 shows the source of aculeatiols, a strain of bacteria called Aspergillus aculeatus. These compounds were successfully isolated from the strain Aspergillus aculeatus during our ongoing research on bioactive compounds derived from this genus. Compound 1 has been proposed as a lovastatin analogue featuring a γ-lactone moiety at the C8 side chain, highlighting its structural relevance to existing lipid-lowering agents [13].
Figure 1: Aspergillus aculeatus
The motivation for synthesizing compounds 1-3 lies in their promising implications for lipid metabolism regulation. Given the established role of lovastatin derivatives in modulating lipid levels, compounds 1-3 may exhibit similar or enhanced bioactivities, making them valuable candidates for further pharmacological evaluation.
As shown in Figure 2, these are the three molecules whose synthesis route will be proposed in this study: aculeatiols A-C. By developing efficient synthetic routes for these compounds, we aim to facilitate comprehensive studies on their biological effects, particularly in the context of lipid uptake and synthesis modulation. The successful synthesis of compounds 1-3 will not only contribute to the understanding of their structure-activity relationships but also pave the way for potential therapeutic applications in managing lipid-related disorders. Thus, this research endeavour is positioned at the intersection of organic synthesis and medicinal chemistry to advance the discovery of novel bioactive agents.
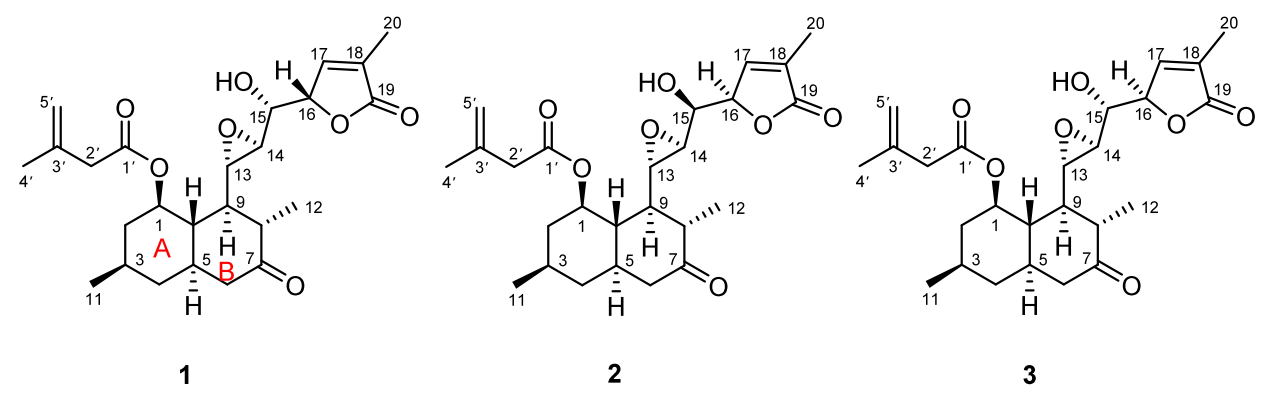
Figure 2: Aculeatiols A-C
To further explore the potential of these novel compounds, we propose a total synthesis approach for Aculeatiols A−C. Our synthetic strategy focuses on several key elements: Robinson Annulation: This reaction is central to constructing the core ring structure, providing a crucial step in building the complex molecular framework of the target compounds. Olefin Metathesis: Employed for carbon skeleton elongation, this reaction helps form the longer carbon chains necessary to synthesise the compounds. Asymmetric Synthesis: Our approach also considers stereochemistry, aiming for an enantioselective synthesis to ensure the desired chiral centres are accurately formed. While we have outlined theoretical steps and identified critical reagents and conditions, further optimization of the purification strategies is necessary. Our objective is to develop a practical and efficient synthesis route that not only produces these new Lovastatin analogs but also contributes valuable insights into their structure-activity relationships. In summary, this study focuses on the total synthesis of Aculeatiols A−C, aiming to provide a solid foundation for future research on these compounds. Our work represents a step forward in the field of statin analog synthesis and offers a practical approach for exploring the potential of these novel molecules.
2. Retrosynthesis Strategy-Robinson annulation as the core of the retrosynthetic strategy
The retrosynthetic strategy employed here is a well-structured and efficient approach. In the subsequent total synthesis based on this method, the target molecules can be synthesized more effectively with a focus on stereochemistry. This strategy hinges on four key reactions: (1) esterification, (2) Robinson annulation, (3) Sharpless asymmetric epoxidation, and (4) olefin metathesis.
Figure 3 shows the core retrosynthesis route of this experiment. Initially, esterification is used to cleave the ester group. Next, Robinson annulation is applied to open the ring, thereby simplifying the molecular structure. The epoxy group is then stereoselectively introduced via Sharpless asymmetric epoxidation. Finally, olefin metathesis, one of this synthesis's most commonly utilized reactions, is employed to facilitate the overall process further.
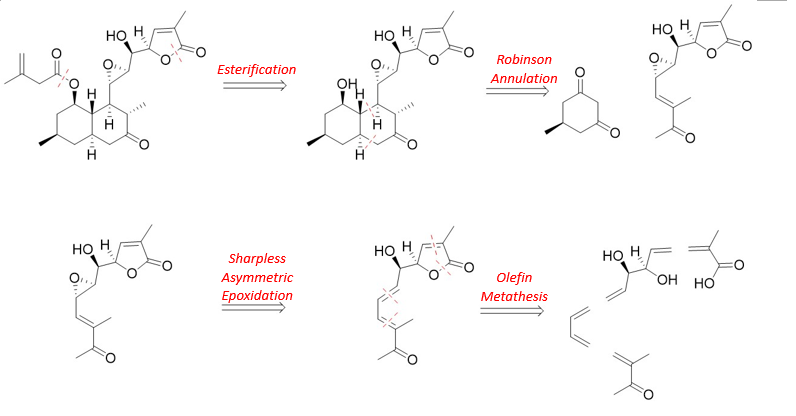
Figure 3: Retrosynthesis of Aculeatiols
3. Total synthesis of Aculeatiols
3.1. Overview of the total synthesis of Aculeatiol molecules by Robinson annulation
Figure. 4 presents a complete synthesis route with Aculetiols B as an example.
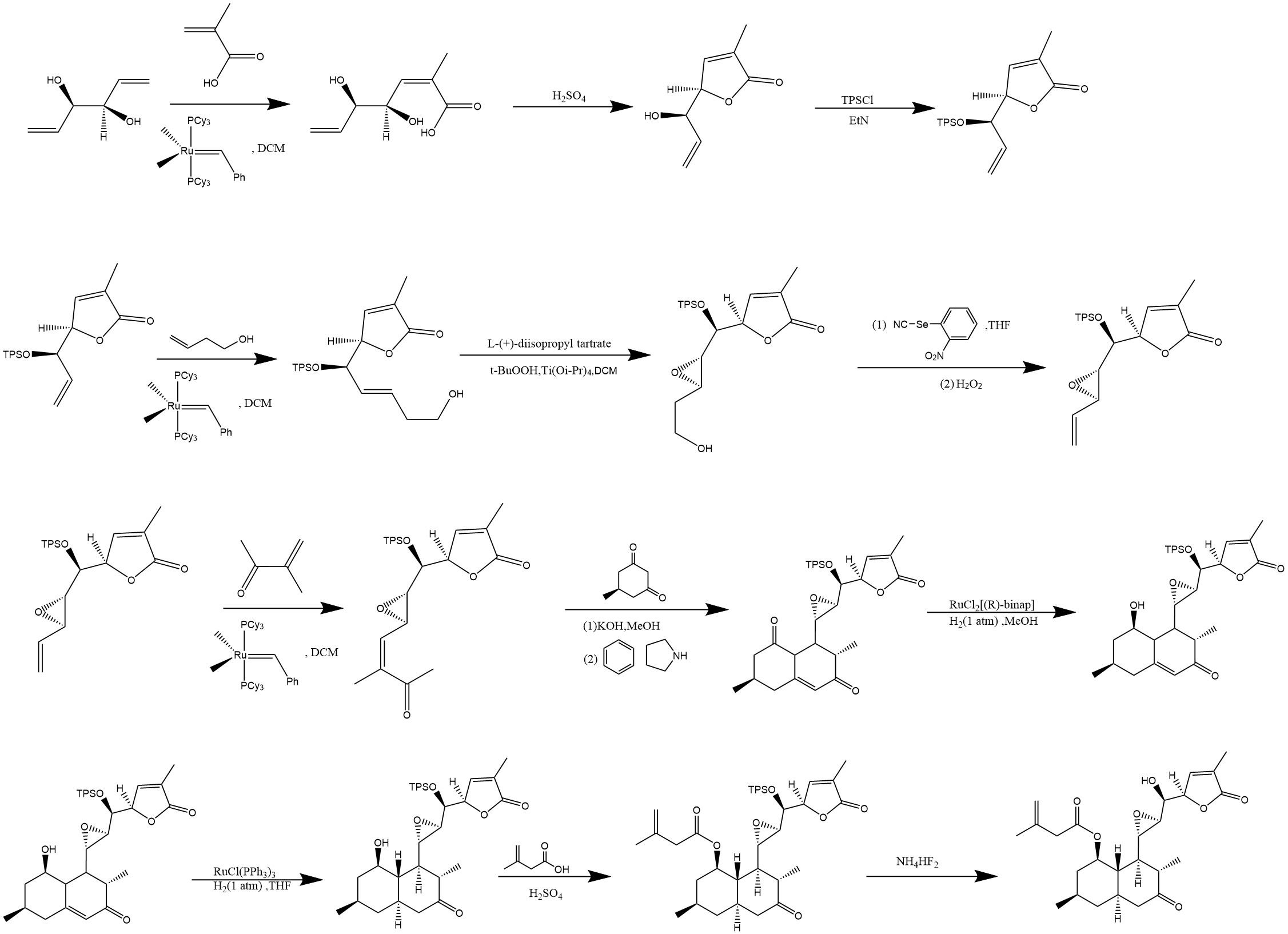
Figure 4: Total synthesis of Aculeatiols
3.2. The total synthesis of Aculeatiols steps
3.2.1. Stereochemistry analysis
Figure 5 shows the stereochemical analysis of the target molecule, which indicates all the stereocenters in the target molecule. There are 10 stereocenters and 4 rings in this structure. Knowing this information is helpful for subsequent total synthesis analysis.
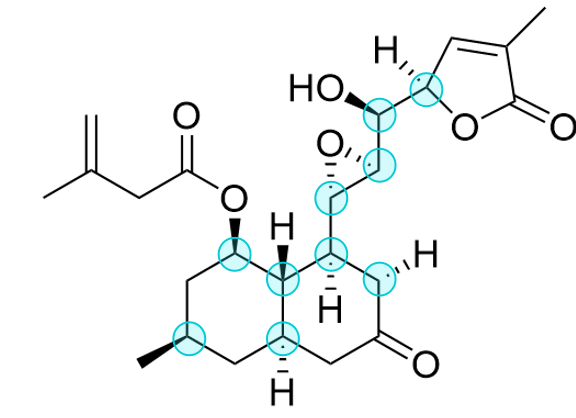
Figure 5: Stereochemistry analysis
3.2.2. Chiral pool
The chiral pool concept refers to using simple, naturally occurring chiral compounds as starting materials for the synthesis of more complex molecules, incorporating stereochemical considerations from the outset. In this approach, three different spatial configurations of the starting materials are employed to facilitate the synthesis of three distinct target molecules.
This method offers a significant advantage, integrating stereochemical control into the reaction design. For instance, when synthesizing compound 1, the high symmetry of the starting material enables the exploration of various chiral versions, enhancing both the selectivity and stability of the reaction. By carefully controlling the stoichiometry and choosing an appropriate substrate for subsequent olefin metathesis, it is possible to improve both the selectivity and yield of the reaction significantly. Thus, employing highly symmetric compounds proves to be critical in this synthesis.
Additionally, for compounds 1 and 2, it is advantageous that the stereochemistry of the chiral centres does not affect the outcome of the olefin metathesis, as either configuration leads to the desired product. Specifically, compound 1 possesses two chiral centres, both in the S configuration, while compound 2 has two R-configured chiral centres. However, in the case of compound 3, the presence of both R and S configurations among the chiral centres may reduce the reaction yield. Theoretical analysis suggests that this reduction could be as high as 50%, owing to the requirement for the olefin metathesis reaction to align with the R configuration of one of the chiral centres in the substrate.
Figure 6 shows 3 reaction initiators corresponding to 3 molecules with different structures.
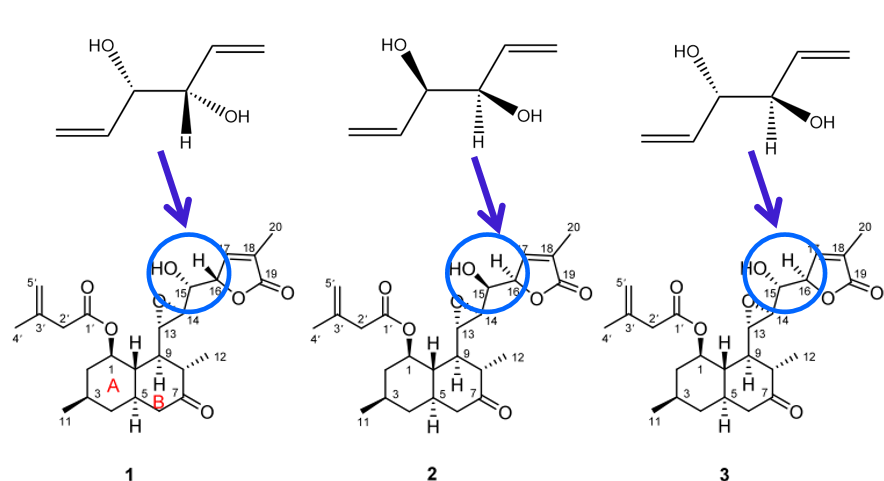
Figure 6: The application of chiral pool
3.2.3. Total synthesis
Figure 7 is the first step in total synthesis. A suitable substrate is first selected for the olefin metathesis reaction. An esterification reaction is conducted to obtain the initial cyclic lactone structure. Finally, the hydroxyl group is protected to prevent it from participating in undesired reactions in subsequent steps.
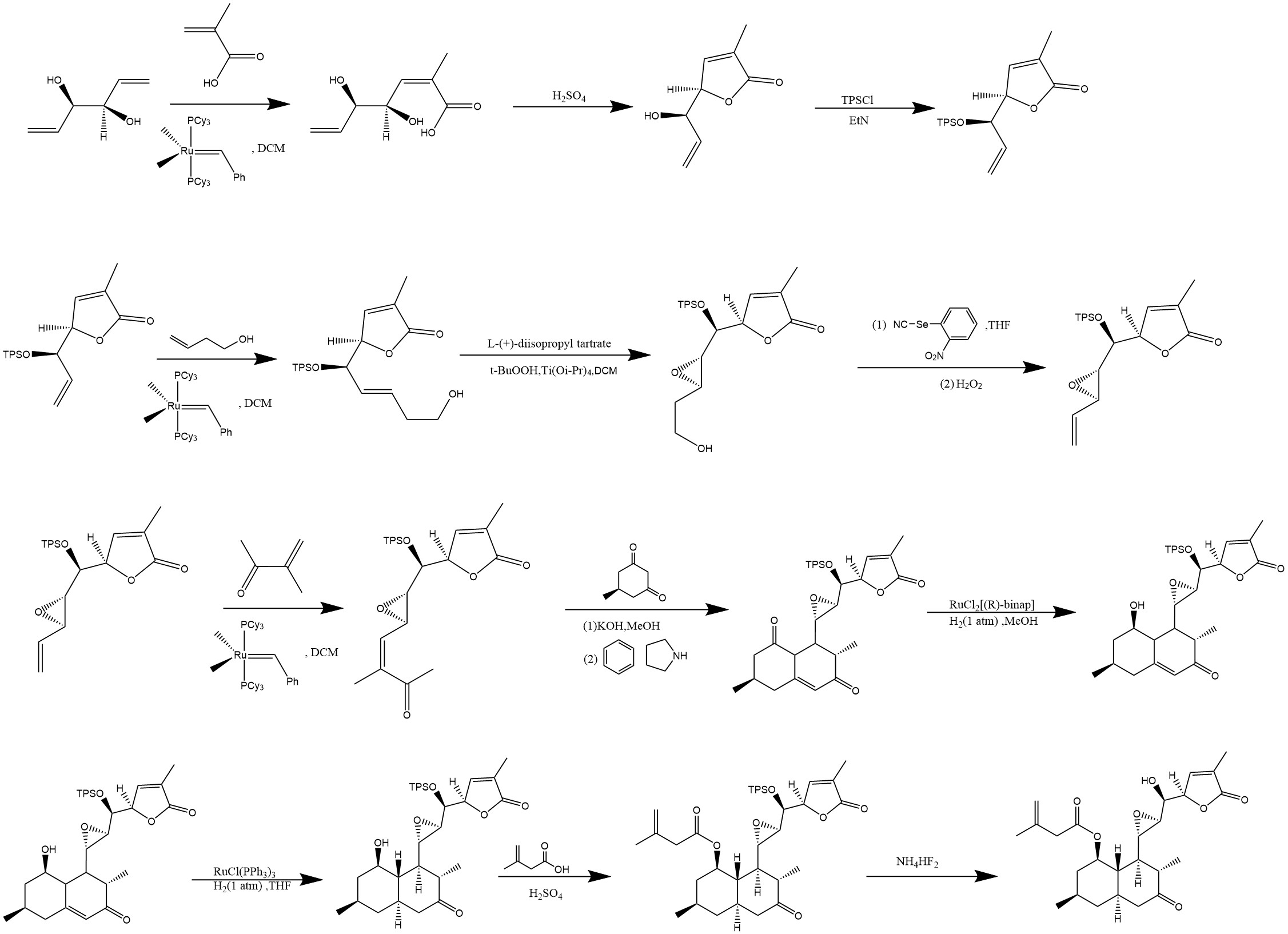
Figure 7: Step 1 of total synthesis
Figure 8 is the second step in total synthesis. The next step involves extending the carbon chain by forming a carbon-carbon double bond through the olefin metathesis reaction. Following this, Sharpless asymmetric epoxidation is employed using the L-(+) configuration of the catalyst to ensure the formation of the desired two stereochemical bonds, both oriented below the plane. Finally, Grieco dehydration, a mild reaction, is used to obtain the carbon-carbon double bond structure, thereby facilitating the subsequent reaction.
Figure 8: Step 2 of total synthesis
Figure 9 is the third step in total synthesis. First, the precursor of the final ring structure is produced through olefin metathesis. Next, the most critical reaction in this total synthesis, Robinson annulation, is employed to construct the pivotal ring structure. The resulting double-ring structure is noteworthy, as the target molecule contains a very stable, nearly planar double-ring saturated alkane structure. This stability allows the molecular framework synthesized by Robinson annulation to adopt a stable planar configuration, reducing the need for extensive stereochemical selection. As a result, the desired structure can be obtained with a higher probability. Finally, asymmetric synthesis is applied to refine this complex ring structure, leading to the target molecule. Noyori asymmetric hydrogenation is used to reduce the carbonyl group, selectively converting it to a hydroxyl group within this stereochemical framework. Due to its conjugative effect, the other carbonyl group remains more stable and less reactive.
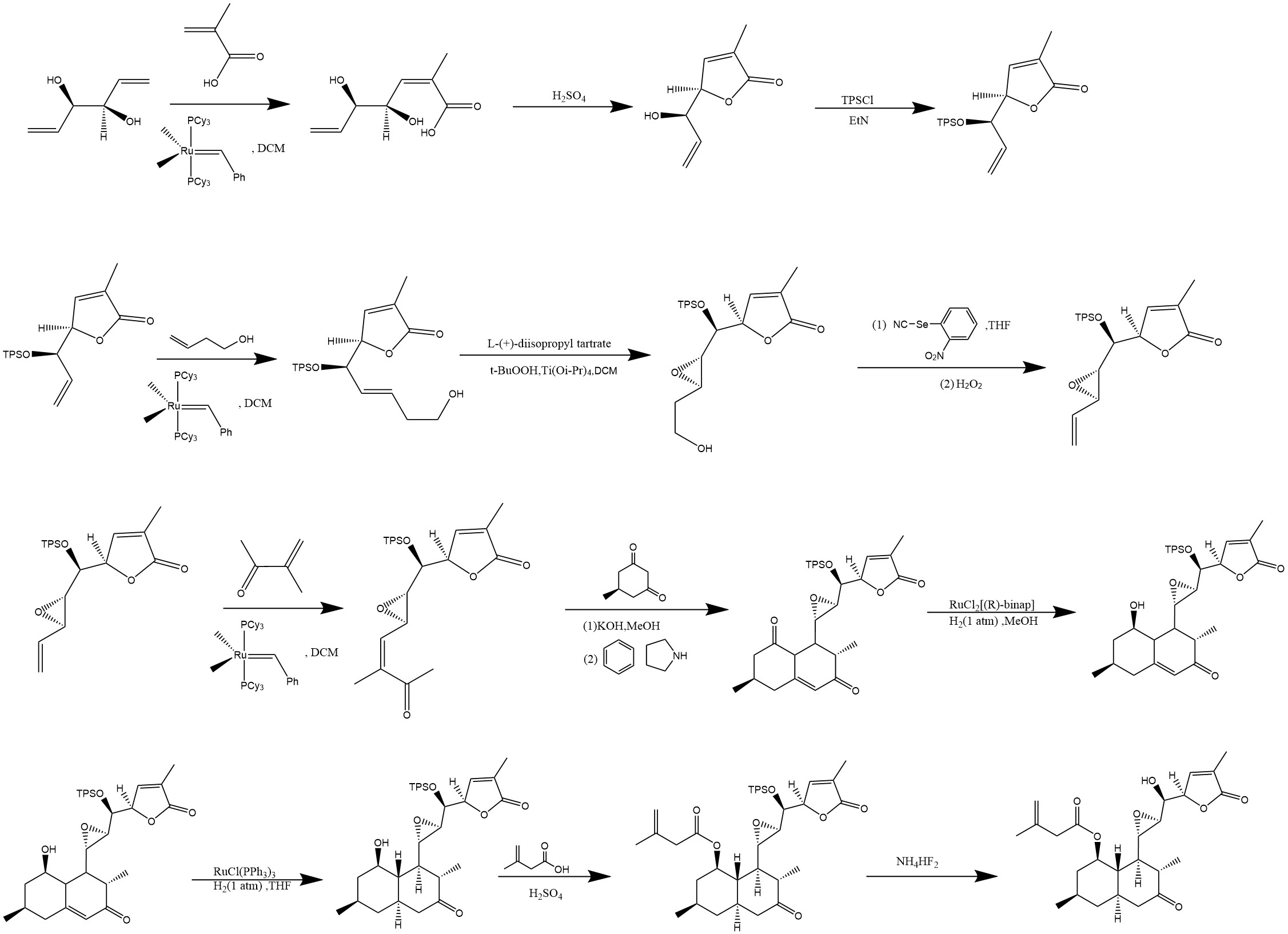
Figure 9: Step 3 of total synthesis
Figure 10 is the fourth step in total synthesis. The carbon-carbon double bond is now addressed by reducing it through homogeneous hydrogenation. Following this reduction, an acid esterification reaction is conducted to form the new molecule. Finally, the hydroxyl group is deprotected to yield the target molecule. This summarizes the total synthesis process of Aculeatiols.
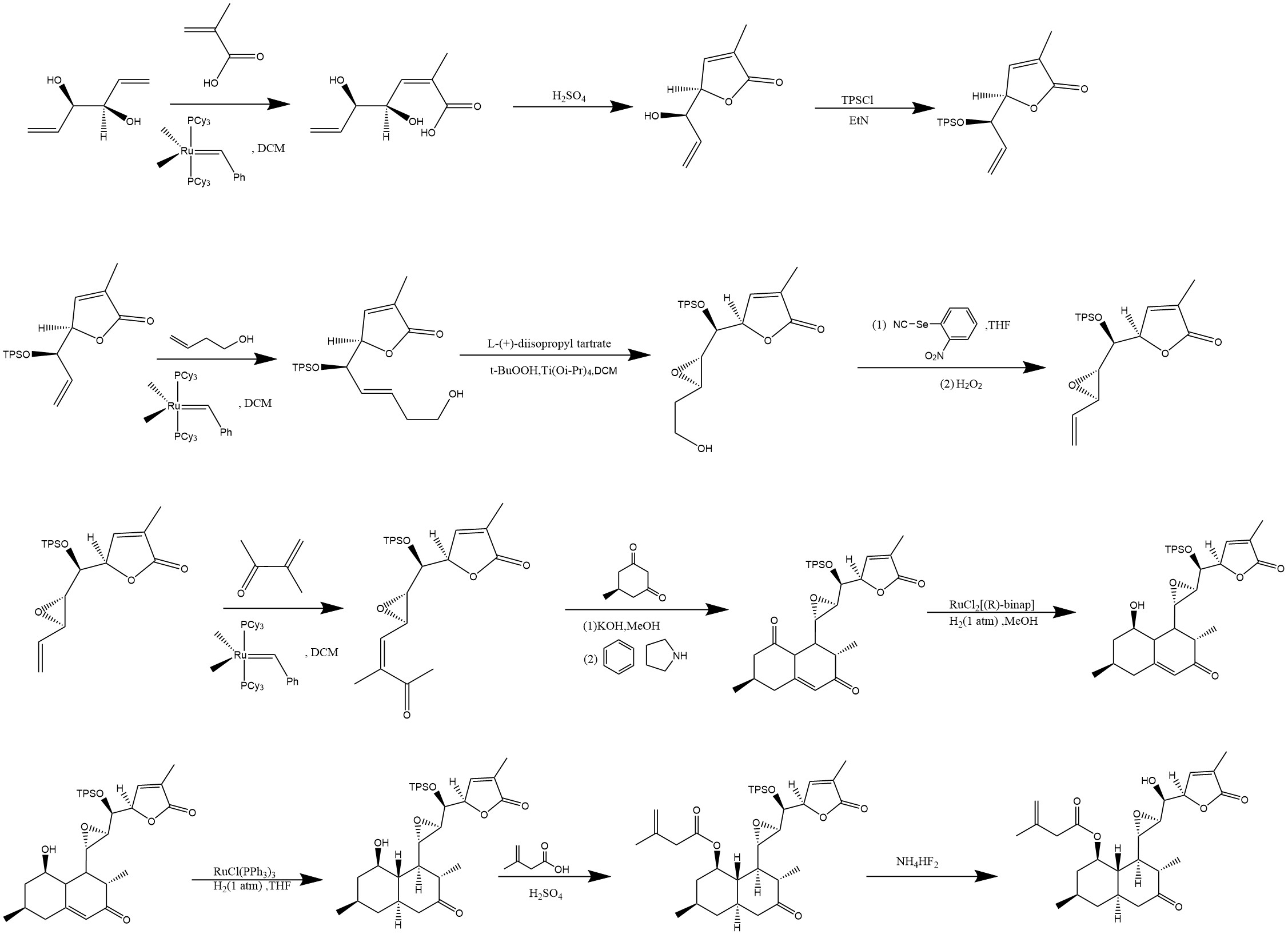
Figure 10: Step 4 of total synthesis
4. Conclusion
This study offers a comprehensive analysis of the total synthesis of Aculeatiols A-C, focusing on the strategies and methods utilized throughout the synthetic process. Initially, a linearization analysis of the molecular structure was performed, leading to the identification of 35 potential structures. From these, the most suitable linearized structure for synthesis was chosen. The Robinson cyclization reaction was a pivotal step in constructing the polycyclic framework of the target molecule.
A detailed stereochemical analysis identified 10 stereocenters and 4 ring structures in the target molecule, critical for determining the subsequent synthetic strategies. The concept of a "chiral pool" was introduced to optimize the synthesis, utilizing simple chiral compounds as starting materials. Key steps included the selection of appropriate substrates for olefin metathesis, followed by esterification to form the first cyclic lactone structure and protecting hydroxyl groups to prevent side reactions in later stages. Overall, these strategies provided an efficient methodology for synthesising complex natural products, highlighting innovation and advanced practices in synthetic chemistry.



