1. Introduction
Fouling, the undesirable accumulation of substances such as scale, sediment, or biological organisms on surfaces and equipment poses significant challenges across various industries, impacting efficiency, safety, and operational costs. This issue is especially prevalent in aquatic environments, where surfaces such as ship hulls, pipelines, and subsea equipment become coated with marine microorganisms, algae, and marine barnacles [1]. It significantly increases frictional drag on ships, leading to increased fuel consumption and operational costs, thereby underscoring the critical need for effective fouling management strategies. [2] In addition to marine biological fouling, the deposition of asphaltenes presents a significant threat to petroleum processing industries, leading to increased cleaning and maintenance costs, as well as significant downtime. Ignoring wax deposits inside oil pipelines can also have serious consequences. If not cleaned up in time, reduced flow rates may lead to the complete clogging of the pipeline, eventually rendering it unusable [3]. Similarly, biofilms developing on medical devices represent a significant concern in the medical field. [4] Their ability to disperse single cells and clusters significantly increases the risk of microbial dissemination within the host, leading to a heightened risk of infection [5]
Different types of fouling can cause substantial economic damage. For instance, data indicates that the US Navy alone incurs an estimated cost of $1 billion annually in addressing marine biofouling issues [6]. Additionally, the cost of well work-overs to address asphaltene deposits can reach as high as a quarter of a million dollars each time [7] Besides, bacterial contamination in dairy manufacturing poses a significant problem, affecting product quality, the economic viability of dairy production, and the sustainability of the industry. [8] Thus, it is vital to either inhibit the adhesion and buildup of these substances or ensure their straightforward removal from essential, functional surfaces.
Understanding the mechanical characteristics of different types of fouling aids significantly in developing technologies to address these challenges. Fouling is generally categorized into hard and soft fouling, each distinguished by distinct mechanical properties, such as the Young’s modulus. [9] In marine settings, hard fouling includes the deposition of calcareous organisms such as barnacles and mussels, forming rigid structures with high Young's moduli. This stiffness indicates their resistance to deformation and complicates their removal. Conversely, soft fouling involves organisms like algae and bacterial biofilms, which are mechanically flexible with low Young’s moduli, potentially allowing for easier removal [10].
Due to recent environmental concerns and policies, a number of effective anti-fouling coating materials have been banned. For instance, legislation has prohibited certain highly effective anti-fouling paints, specifically those containing tributyltin oxide, and has implemented stricter evaluation and regulatory measures for the use of alternative biocides [11] Consequently, the development of bio-inspired surfaces, along with other innovative non-toxic coatings, is essential to address these challenges effectively.
There are numerous natural strategies to prevent hard and soft fouling. For example, while surface topography alone may not effectively prevent biomass accumulation [12], decapod crabs employ a more robust method involving the physicochemical modification of their micropatterned surfaces through epidermal secretions [13] Similarly, PDMS, a silicone material with low surface energy, offers an avirulent option to traditional biocide paints. Despite PDMS is actually not inherently anti-fouling and can accumulate both hard and soft fouling [14], incorporating the unique pattern of shark skin has been proven to significantly enhance its ability to deter organism settlement [15].
Based on natural antifouling strategies, various synthesis methods have been developed that move beyond traditional techniques for creating micro- to nanoscale textures. These methods include several indirect manufacturing techniques designed to replicate naturally occurring topographies and are categorized into four main types: molding, lithography, surface corrugation, and chemical etching. [16] Additionally, synthesis strategies involving natural chemical antifoulants derived from marine organisms such as corals and sponges, as well as those targeting quorum sensing processes in bacteria, have shown significant effectiveness in combating marine biofouling [17]. Compounds like butenolide have demonstrated promising antifouling properties, with some formulations maintaining efficacy over extended periods [18].
Current antifouling surfaces face significant limitations, including poor durability, high costs, and reduced effectiveness against a range of fouling organisms [19]. Many coatings degrade quickly, requiring frequent replacement, which increases maintenance costs and environmental impact [20]. Although environmentally friendly alternatives are being developed, they often lack the durability and efficacy of traditional biocide-based solutions, making it difficult to balance effectiveness with sustainability [11].
Developing a durable surface that can prevent both soft and hard fouling remains a major challenge, but an interdisciplinary approach that combines physical, chemical, and biological strategies offers a promising solution. [19] For instance, manipulating surface textures with micron or nanometer roughness and creating superhydrophobic designs inspired by lotus leaves have shown effectiveness in reducing fouling. [21] Advanced chemical coatings, such as fluoro group polymers or silicone-based materials, help lower surface energy to prevent adhesion, while embedded antimicrobial agents specifically target soft fouling. [22] Innovative methods, like kirigami-inspired superhydrophobic sheets that dynamically adjust their structures, also demonstrate potential in minimizing adhesion across multiple pollutants. [23] By integrating these diverse strategies, it is possible to develop versatile, cost-effective antifouling technologies suitable for various applications.
2. Fundamentals of wettability, surface energy and elastic modulus
Understanding wettability, surface energy and elastic modulus of materials is essential for developing materials that mitigate soft and hard fouling. [19] These properties can also help quantify the anti-adhesive properties of surfaces, and can critical to help understand the mechanism of action of different bioinspired antifouling coatings [24]. Understanding how these fundamental characteristics influence antifouling performance is crucial for developing more effective anti-fouling coatings for a wide-variety of applications.
Surface wettability is a measure of the intramolecular forces acting between the solid and liquid surfaces. One of the simplest, and most effective measures for quantifying surface wettability is the so-called Young’s contact angle [25],. When a liquid and a solid come into contact, three distinct phases are present: the solid, liquid, and gas phase. [26] The Young’s contact angle can be readily obtained by a simple force or energy balance and is given as:
\( {γ_{SG}}={γ_{SL}}+{γ_{LG}}cos(θ) \)
Based on the Young’s contact angle with water, we can characterize surfaces in to four distinct types [27]. If the water contact angle on a surface is less than 10º, the surface is considered superhydrophilic, exhibiting extremely high affinity for water. When the contact angle is between 10º –90º, the surface is characterized as hydrophilic. [28] If the water contact angle ranges from 90º – 150º, the surface is classified as hydrophobic, showing a reduced tendency to interact with water. Once the water contact angle is > 150º, it is considered superhydrophobic, indicating an extreme resistance to water adherence. [29] (See Fig.1)
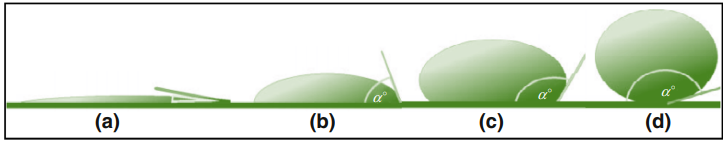
Figure 1: (a) Superhydrophilicity, (b) hydrophilicity, (c) superhydrophobicity, and (d) superhydrophobicity [30] of different surfaces. Here a is the contact angle
However, the interaction of liquid with complanate solid surface typically represents an idealized scenario. In order to depict how liquids engage with textured or rough surfaces accurately, researchers have developed models such as the Wenzel and Cassie-Baxter states. The Wenzel state occurs when a liquid thoroughly wets the surface, filling in all the grooves and contours, which increases the contact area of liquid with surface and generally results in higher adhesion and a reduced contact angle [31]. On the other hand, the Cassie-Baxter state occurs when the liquid only partially wets the textured solid, leaving air pockets trapped underneath the contacting liquid droplet. [32] In recent years, extensive research has focused on the wetting transition between these two states. This transition shows reversibility as soon as both states reached thermodynamic equilibrium by altering parameters like temperature and pressure, or through the application of external forces like electric fields [33]. Additionally, an intermediate state exists that partially fills the voids on the surface, presenting a higher energy barrier that slows the conversion from meta-stable Cassie-Baxter state to stable Wenzel state. [34] (See Fig.2)
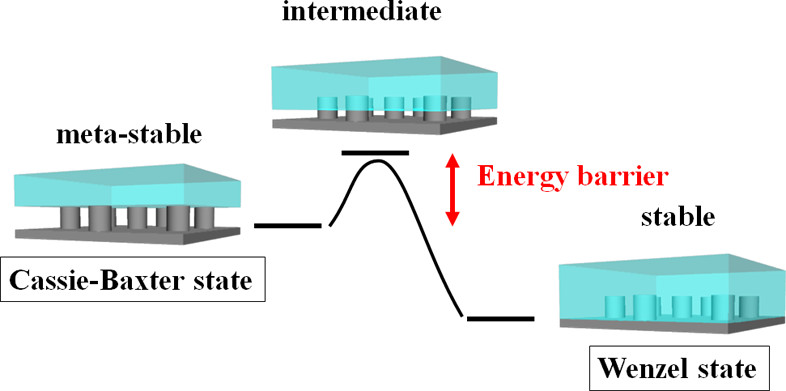
Figure 2: A model explicitly illustrate that the energy barrier in the wetting transition from Cassie-Baxter state to Wenzel state is influenced by the interplay of the inherent energy barrier and external forces [35]
Superhydrophobicity is typically associated with the Cassie-Baxter state. Superhydrophobicity was discovered for a large number of natural surfaces such as the lotus leaves and the legs of the water striders, and has several practical uses in reducing ice adhesion and enhancing self-cleaning capabilities. [36] On the other hand, some wool fabrics can be made superhydrophilic in order to enhance their ability to absorb water and effectively remove sweat from the body [37] Thus, surfaces with varying wettability properties exhibit unique strengths, interactions with solids and liquids, potentially offering insights for the development of innovative antifouling surfaces.
Surface energy refers to the energy required to create a new surface, encapsulating the disruption of intermolecular bonds within a material. [38] Surface energy fundamentally influences the wettability of materials, where higher surface energies enhance a material's ability to bond with liquids, while lower surface energies reduce the likelihood of a material interacting with other liquids or solids, making such surfaces very useful for reducing bio-contamination [39]. Notably, studies on materials like graphene have demonstrated that alterations in surface energy, coupled with electrostatic interactions between graphene and bacteria, lead to significant enhancement in a materials antifouling properties [40] These interactions lead to a foul release effect, which is crucial for foulant removal. As an example, previous work has shown that negatively charged bacteria in water are repelled by the graphene coatings [41].
Similarly, fracture mechanics offers valuable insights into the material removal process by studying crack propagation, focusing on how tiny defects act as stress concentration points to encourage crack growth and ultimately enable material detachment. [42] The field categorizes three main crack-opening modes: peeling, in- and out-of-plane shear [43] (See figure 3) Typically, adhesive joints are most likely to fail via peeling (Type I), as this mode requires less energy compared to failures in shear (Types II and III) [43].
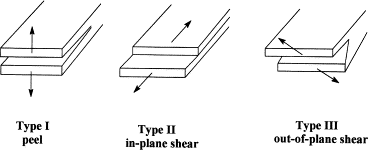
Figure 3: Fracture mechanisms for failure of a joint
As shown in Figure 4, a homogeneous plate contains a flaw. The equation for the critical stress \( ({σ_{c}} \) ) required to propagate a crack in the plate under uniaxial stress has been formulated as [44]:
\( {σ_{c}}=\sqrt[]{E{G_{c}}/πa(1-{υ^{2}})} \)
Where \( E \) represents the elastic modulus, \( {G_{c}} \) is Griffith's critical fracture energy per unit area, \( a \) denotes half the crack length, and \( υ \) is Poisson's ratio.
When the stress reaches the critical value, the crack begins to expand. At this critical time, the critical pull-off force \( Pc \) , acting over an area \( A=π{a^{2}} \) , is given by:
\( \sqrt[]{\frac{πEG{c_{a}}{a^{3}}}{1-{ν^{2}}}} \)
It’s Kendall that followed Griffith’s fracture analysis a few years later, modeled the adhesivity of elastomer substrates. He deduced the critical pull-off force for a thin elastomer film where the disc radius is less than the elastomer film size. It consists of a rigid disk and a rigid substrate that are sandwiched with an elastomeric coating [45] (see Figure 4). The critical pull-off force \( Pc \) is expressed as [45]:
\( Pc=π{a^{2}}\sqrt[]{\frac{2GcK}{t}} \)
where \( K \) represents the normal force and \( t \) notes the coating thickness.
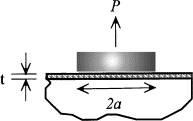
Figure 4: A rigid disk applies a pull-off force \( P \) to an elastomeric coating with thickness \( t \) , adhered to a rigid substrate, across a contact radius \( a \)
For the elastomeric coating with great thickness where the thickness \( t \) is much greater than the contact radius \( a \) , Kendall evolved the nether calculation:
\( \sqrt[]{\frac{2πEGc{a^{3}}}{1-{ν^{2}}}} \)
This equation demonstrates that thickness is not a significant index anymore, but the elastic modulus ( \( E \) ) and critical surface energy ( \( Gc \) ) play prominent roles. This indicates that PC primarily depends on the product of \( E \) and \( Gc \) . In the case of materials sharing the same modulus of elasticity, PC varies with Gc, and for those with identical Gc, Pc changes with E. These relationships can be articulated as follows:
\( Pc∝\sqrt[]{E{G_{c}}} \)
\( Pc∝\sqrt[]{E} \)
\( Pc∝\sqrt[]{{G_{c}}} \)
The three equations illustrate that \( Pc \) has proportional relationships with the square root of either \( EGc \) , \( E \) , or \( Gc \) . This relationship indicates that materials with lower values of \( E \) and \( Gc \) will have reduced critical adhesion strengths, enhancing their antifouling properties. According to the Griffith theory of fracture, these equations are key in predicting the antifouling effectiveness of surfaces based on their elastic moduli and surface energies [46]. This insight is valuable for designing surfaces that resist biological fouling, which is crucial in many applications such as marine coatings and medical devices.
3. Natural antifouling surfaces
Many animals and plants possessed exceptional antifouling properties, with significantly different contaminant removal techniques among species. Three antifouling approaches mainly include (1) non-chemical techniques, which primarily involve specialized surface topographies [16] and dynamic shedding effects [47] or instability [48]; (2) chemical techniques, which utilize natural antifoulants [49] or chemically modified surfaces including zwitterionic coatings [50] and mucus-like hydrogels [51]; and (3) hybrid techniques that combine elements of both chemical and non-chemical methods, such as slippery liquid-infused porous surfaces [52]. These strategies exhibit the innovative ways of addressing biofouling by nature, offering valuable insights for developing advanced antifouling technologies.
To probe into non-chemical antifouling techniques, it has concentrated on the detailed topographies of natural surfaces as well as the creation of their replicas through biomimetic (BM) or bioinspired (BI) approaches. [53] Investigators have extensively studied the surface topographies of various natural organisms, concerning seeds [54], mangrove leaves [55], and shark skin [56]. These specific cases will be discussed in detail in the following sections, highlighting the unique surface structures contributing to their antifouling properties.
Mangroves, which grow in the intertidal zones, are especially fascinating for antifouling research. Despite being regularly submerged in seawater, their leaves remain remarkably free of fouling, making them an excellent subject for exploring natural antifouling mechanisms. Some factors may attribute to these exceptional mechanisms, including low surface wettability, the presence of the natural antifoulant oleanolic acid, and effective mechanisms for post-settlement detachment [57] Although these factors are observed in all mangrove species, many do not show the same level of antifouling efficacy. [57] Thus, it is proposed that the surface morphology of S. apetala leaves is the primarily responsible for driving the exceptional antifouling properties [55]. To test this hypothesis, a PDMS replica was created with a ridge-like surface topography, characterized by an interval and height of 5 μm between each ridge. Those ridges appear with no specific alignment or orientation (see Figure 5). Comparative analyses reveal that this replica displays significantly more resistant to adhering tubeworm Hydroides elegans than a glaze PDMS surface lacking any topographical features [55].
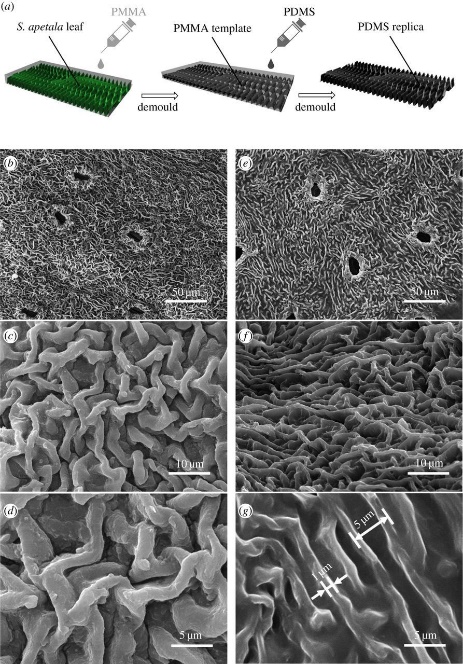
Figure 5: (a) A schematic representation of the process used to create PDMS replicas of S. apetala leaves. (b–d) show scanning electron micrographs of an S. apetala leaf surface and compare these with images of the PDMS replica (e-g)
The biofouling defenses of land plant seeds have only occasionally been studied in detail. Charles Darwin was among the early explorers to study this phenomenon, investigating how seeds and fruits drift across oceans during his 19th-century voyage on the HMS Beagle [58]. These seeds can stay afloat for up to 14 months, influenced by factors such as earth's movements, ocean currents, wind-surface water friction, and variations in salinity and temperature.
To investigate the remarkable antifouling capabilities of various seeds, field experiments were conducted on the drifting seeds from 43 different species collected from various global locations. Despite the presence of barnacles in the testing area, six of the seed species remained free from barnacle fouling after 12 weeks. [54] Four of these species: Coccothrinax borhidiana, Sapindus saponaria, Ipomoea alba, and Erythrina berteroana, feature surface microstructures with irregularities < 5 μm. The remaining Licuala spinosa and Acoelorrhaphe wrightii possess typical honeycomb structures with widths between 15.2 and 19.0 μm and lengths between 21.7 and 28.0 μm. The roughness (Ra) of these antifouling surfaces ranges from 0.36 μm to 2.09 μm. (See figure 6) Taking these findings as inspiration, bio-inspired surfaces were created using silicon infused with hollow nanoparticles. Undergoing field tests for 12 weeks, these surfaces showed a significant ease of cleaning, indicating weaker adhesion of barnacles to the substrate, which mirrors the antifouling characteristics observed in the seeds studied [54].
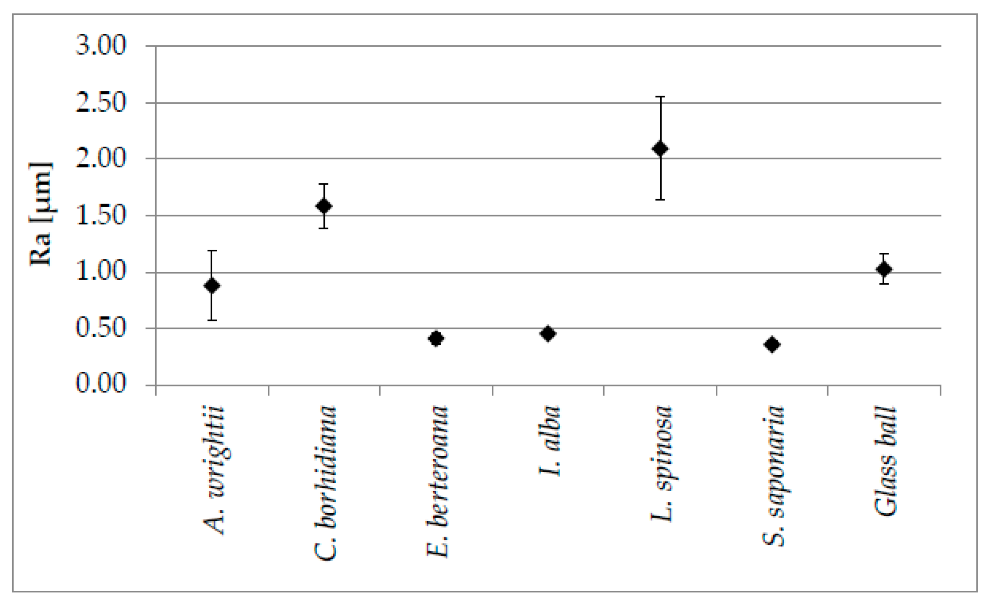
Figure 6: The mean roughness (Ra) and standard deviations of profiles from six drifting seed species were analyzed and compared to a reference glass ball using digital elevation models
Shark skin is one of the most extensively studied natural surfaces for its antifouling properties. Drawing inspiration from this, a antifouling surface known as Sharklet was developed for commercial use [56]. This surface features platelet patterns crafted from PDMS using photolithography, which have been shown to eliminate 86% of zoospore settlement compared to glaze surfaces (see Figure 7). Additionally, various parts of the Scyliorhinus canicula (small-spotted catshark) skin exhibit different levels of biofouling resistance. [13] Biomimetic (BM) replicas revealed that the second dorsal and caudal regions of the shark skin demonstrate enhanced antifouling performance in field tests lasting two weeks, with approximately 4% less fouling mass compared to the BM surface of shark head. Research also extended to the eggshell surface of the shark S. canicula, which displayed temporary antifouling properties [59].

Figure 7: Shark skin corresponding synthetic replicas
In addition to the topographical features of a surface, dynamic effects are also effective for the physical removal of contaminants. For example, epithelium shedding is a mechanism used by marine organisms, such as crustose coralline algae, to sweep fouling organisms away from surfaces [47] Dolphins have a different dynamic approach to preventing fouling accumulation. Their skin creates an unstable surface in turbulent water, making it difficult for organisms to settle and attach [48].
Over the past fifty years, substantial data has been collected concerning the surface characteristics of crustose coralline algae. This period has also seen detailed studies of the shedding phenomenon. For two species of Clathromorphum, it has been proposed that epithelial layers are periodically sloughed off, allowing these species to maintain a consistent thickness despite ongoing new cell production at the meristem [60]. Additionally, reports indicate that a delicate white calcareous film, possibly linked to cover cells, has been observed flaking off along with adhered blue-green algae from the Dermatolithon litorale’s surfaces [61].
Four crustose coralline algae species, Lithophyllum okamurai Fosl., Lithophyllum yessoense, Neogoniolithon sp., and Lithothamnium japonicum Fosl., were collected for study. The shedding phenomenon of epithelial layers was specifically found in L. yessoense using Scanning Electron Microscopy after culturing zoospores of Laminaria japonica on the crusts for thirty days. It was notably observed that vegetative surfaces occurred peeling off from crusts, facilitating the removal of L. japonica germlings and other contaminative microorganisms.[47] Vertical section observations through crusts indicated that the new epithelium cells was pushed out by underlying meristems, peeling off the upper epithelium cells. (See Figure 8)
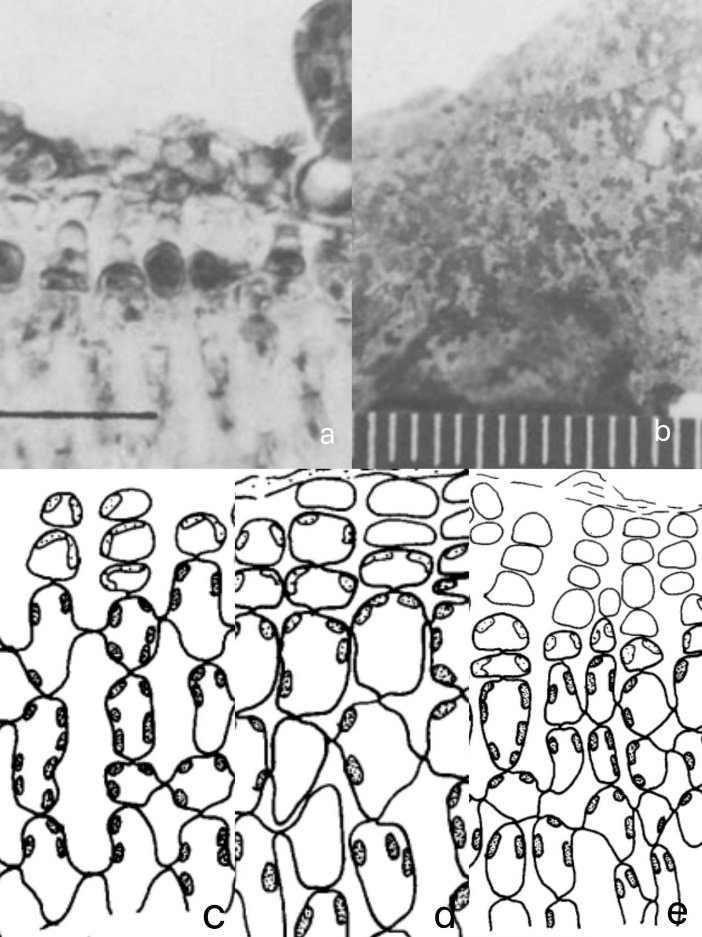
Figure 8: (a) Vertical section photomicrograph of a Lithophyllum yessoense crust, illustrating the detachment of an epithelial layer along with a Laminaria japonica germling. Scale in 50 µm (b) The surface of Lithophyllum yessoense is marked by distinctly visible white patches where the epithelial layer is shedding. Scale in millimeters. (c-e) Vertical sections through the vegetative portion of a Lithophyllum yessoense crust, illustrating the progressive stages of epithelial shedding [47]
Along with surfaces that exhibit the shedding phenomenon, other surfaces perform a dynamic ability to remove contaminants in a different way. For instance, studies on dolphin’s skin have highlighted its capability to reduce drag through small synchronous vibrations in response to external forces. The dolphin's soft skin features arrays of lateral grooves and is underpinned by musculature that facilitates vibration. This movement creates an unstable surface that discourages the settlement of fouling organisms [19].
A variety of chemical antifouling methods are exhibited on natural surfaces. Prominently, natural antifoulants reduce fouling by releasing bioactive compounds from various marine species. Many marine invertebrates, such as sponges and corals, are known to remain remarkably free from fouling organism settlement. This indicates they contain biologically active compounds that inhibit the inhabitation and development of other marine organisms' larvae on their bodies. Researchers have identified several such compounds in marine invertebrates, which are thought to play a significant role in their antifouling mechanisms.
Controlled experiments with Antarctic soft corals, specifically Alcyonium paessleri May and Gersemia antarctica Kukenthal, have demonstrated antimicrobial and antifouling activities. In contrast, the control group, represented by the stoloniferan Clavularia frankliniana Roule, showed no such activity. These findings suggest that these corals depend on ingredients that prevent both microbial attachment and growth. This strategy could be crucial in preventing "surface conditioning" by early successional stage bacteria, potentially limiting later successional fouling stages.
Natural product antifoulants is primarily derived from marine organisms like sponges, corals, and starfish.It encompass a diverse range of compounds, including terpenes, nitrogen-rich compounds, phenols, steroids, and others. [49] Research has been dedicated to identifying antifouling agents from marine invertebrates as well as their commensal microbes in the South China Sea over the years. [62,63] Notable among these are the dihydroquinolin-2-one-containing alkaloids from a fungus associated with the gorgonian Scopulariopsis sp. [63], briarane-type diterpenoids from the gorgonian Dichotella fragilis [64], and resorcylic acid lactones from the fungus Cochliobolus lunatus derived from marine sources [65].
In a comprehensive investigation, 55 natural products combined with corresponding synthesized derivatives, sourced from one sponge, two gorgonians, and five symbiotic fungi gathered from the South China Sea, were evaluated to identify their antifouling properties against the larval settlement of B. amphitrite. [66] The findings revealed that, apart from phenyl ether derivatives showing strong anti-fouling activity, only two other compounds demonstrated significant antifouling effectiveness, a diterpenoid with two furan rings and a 9,11-secosteroid. [66] The activity of all other tested compounds was generally weaker. Among these, the phenyl ether derivatives, particularly the polybrominated diphenyl ether derivative 2,4,6,2',4',6'-hexabromo-diorcinol, showed promising results, suggesting potential as a highly efficient, low-toxicity, and environmentally friendly antifouling agent [66]
Some natural surfaces lack inherent antifouling properties, but advancements in anti-fouling coatings have enabled the enhancement of these surfaces' resistance to biofouling. To tackle biofouling without relying solely on anti-fouling coatings, diverse hydrophilic polymers, such as poly(N-vinylpyrrolidone), zwitterionic polymers, poly (ethylene glycol) (PEG), and poly(2-oxazoline), along with poly (vinyl alcohol), have been exploited. These polymers are employed to construct hydrophilic surfaces that significantly reduce biofouling. Among these, PEG stands out as a biocompatible polymer [67] and has become the gold standard for unfouling applications due to its highly-hydrophilous, innoxious, and anti-protein-fouling properties [68]. However, despite its effectiveness, PEG faces challenges with stability; it is susceptible to rapid autoxidation and degradation in biologically related solutions during reservation and processing at room temperature, particularly in the existence of transition metal ions [69].
Based on these advancements, zwitterionic polymers have emerged as excellent alternatives to PEG. Unlike PEG's amphiphilicity, zwitterionic polymers are exceptionally hydrophilic, boasting a strong hydration layer due to the abundance of ionic groups [70]. The inspiration for using zwitterions against fouling comes from the mammalian cell membrane's external surface, which is rich in phospholipids with zwitterionic headgroups like phosphatidylcholine. This surface predominantly features these zwitterions on the extracellular side of the lipid bilayer, with fewer zwitterions on the cytoplasmic side. The remarkable antifouling properties of a single monolayer of lipid zwitterion are enhanced by its positioning on a hydrophobic layer of hydrocarbon chains, underscoring its potential in biofouling prevention [50].
Mucus is another vital surface modification agent that effectively combats biofouling in many aquatic organisms, such as fish and some amphibians. Fish mucus functions in communication, disease resistance, respiration, as well as ionic and osmotic balance. It also aids in feeding, nest building, reproduction, and excretion [71]. Additionally, fish mucus serves as a protective barrier being like a lubricant that provides a mechanical defense system between the internal and external environments [72]. The antimicrobial properties of skin mucus are due to antimicrobial peptides, an innate immune component essential for early defense in various species. [51] These peptides, part of abundant short polypeptides that occur naturally, possess amphipathic-helical structures that effectively breach and disrupt bacterial phospholipid membranes [73]. Notable examples include pelteobagrin from the skin mucus of the yellow catfish (Pelteobagrus fulvidraco) [74], pleurocidin from winter flounder [75], and parasin found in the epithelial mucosa of catfish (Parasilurus asotus) [76].
Recent research has underscored the powerful antimicrobial properties of fish mucus in protecting fish from the microbial threats in their habitats. The antibacterial effectiveness of skin and mucus extracts from the freshwater fish species C. batrachus and T. mossambicus demonstrated a robust inhibitory effect against a variety of microorganisms, highlighting the essential function of this natural protective barrier. Some studies on Channa striatus also showed its mucus was effective against six different bacterial strains. [69] Moreover, both native (e.g., Catla catla and Labeo rohita) and introduced (e.g., Hypophthalmichthys molitrix and Ctenopharyngodon idella) fish species have exhibited significant antibacterial and antifungal capabilities, further affirming the critical antimicrobial role of fish mucus in diverse aquatic species [66].
The dermatous exocrine glands of amphibians are potent biochemical factories, proficient at producing large quantities of hormones and neuropeptides including thyrotropin-releasing hormone, angiotensins, bombesin/gastrin-releasing peptide, tachykinins, and bradykinins [74]. In a similar vein, the serous glands of Anurans generate a wide variety of gene-encoded antimicrobial peptides. These peptides are crucial for protecting their sensitive skin from pathogenic microorganisms and assisting in the healing of wounds. [77] These peptides effectively and quickly neutralize an array of yeasts, protozoa, fungi, and bacteria by infiltrating and breaking down their plasma membranes or neutralizing their endocellular mechanisms. It substantially prevents the emergence of resistance among the pathogens.
The predominant mechanism through which frog skin antimicrobial peptides (AMPs) operate involves the disruption of the lipid plasma membranes of pathogenic cells. These AMPs, which are generally cationic, bind selectively to the anionic lipids found in prokaryotic membranes, facilitated by long-range electrostatic interactions (See Figure 9). [78] However, their function extends beyond membrane disruption. Cationic AMPs also have the capability to interfere with intracellular targets and halt critical cellular functions, including DNA and protein synthesis, protein folding aided by chaperones, enzymatic processes, and key stages of cell division and wall construction. [79] Despite the diversity in their structural and functional attributes, characterized by variations in hydrophobicity, length, sequence, amino acid composition, membrane-bound conformation and amphipathicity, the comprehensive effectiveness of these natural AMPs highlights their potential as bio-anti-fouling agents, effectively preventing microbial colonization and biofilm development on both biological and artificial surfaces [80].
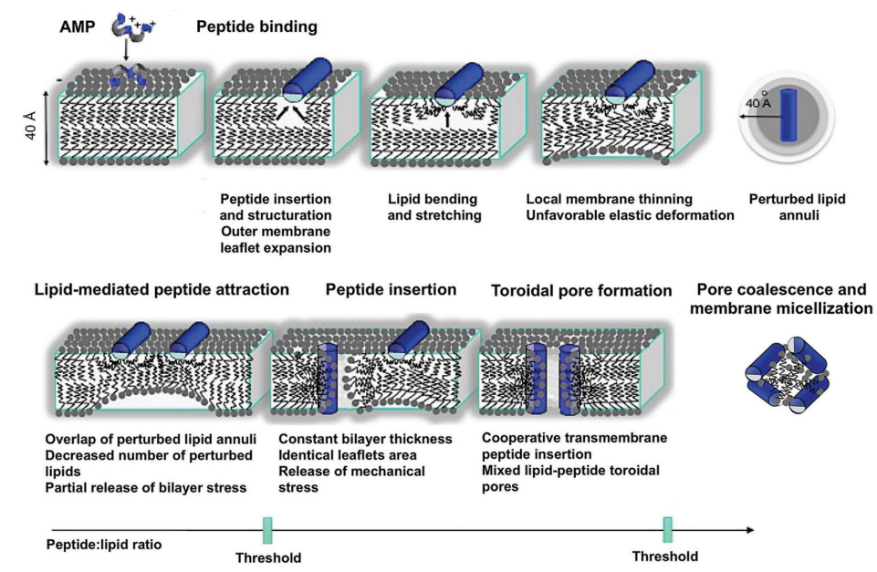
Figure 9: Alpha-helical antimicrobial peptides from frog skin disrupt bacterial membranes through a multi-step process. Initially attracted by electrostatic interactions, the peptides bind to the membrane and form amphipathic helices, inducing membrane strain. As peptide concentration increases, they reorient and form toroidal pores, ultimately causing membrane disruption when these pores merge [78]
Both physical and chemical properties play crucial roles in natural antifouling surfaces and integrating them can enhance their effectiveness. In 2011, Wong et al. pioneered the development of a Nepenthes pitcher-inspired slippery liquid-infused porous surface (SLIPS), utilizing nano/microstructure substrates to secure the infused lubricating fluid, a concept inspired by the Nepenthes pitcher plants. [81] Notably, the waxy zone of these pitchers features platelet-shaped aldehyde crystals that protrude perpendicularly from the surface. [82] These crystals not only interfere with the adherence of insects by contaminating their adhesive pads but also react with the insects' adhesive secretions, creating a substance that further hinders attachment, enhancing the plant's antifouling properties [83].
In addition to Nepenthes pitcher plants, the earthworm also consists of a typical slippery liquid-infused porous surface. They have a unique self-lubricating principles due to their sophisticated epidermal glands which could continually generate mucus in the presence of external mechanical stimuli [84] and rough skin with the composition of macroscopic annuli and micro ripples for stabilizing the mucus as a thick slippery layer.(See figure 10) [85] Furthermore, the previously discussed methods have shown promise in fluid-based environments but may prove inefficient in solid-based contexts due to the potential for strong physical friction to damage fine microstructures or displace lubricants stabilized within the substrates. Addressing this issue, earthworms present a potential solution, as they naturally inhabit solid-based environments. Therefore, it is feasible to emulate the lubricating mechanisms of earthworms, with particular focus on the integration of rough interfacial morphology and adaptive “secretion” capabilities.
In addition to the Nepenthes pitcher plants, earthworms also exhibit a remarkable self-lubricating mechanism enabled by their sophisticated epidermal glands. These glands continuously produce mucus as a response to additional mechanical stimuli (Ren et al., 2001), which is then stabilized on their coarse skin. This skin structure helps maintain a thick, slippery layer of mucus, effectively reducing friction as they move through soil (See Figure 10) (Gao et al., 2010). Previous methods discussed for fluid-based environments might falter in solid-based surroundings due to the strong physical friction that could damage the delicate microstructures or displace the lubricants embedded within the substrates. However, earthworms thrive in such solid-based environments, making their lubrication strategy particularly interesting. By focusing on the interplay between their rough interfacial morphology and their adaptive secretion capabilities, it may be possible to develop new anti-fouling surface with lubricating mechanisms inspired by earthworms that are effective even in challenging solid-based contexts [86].
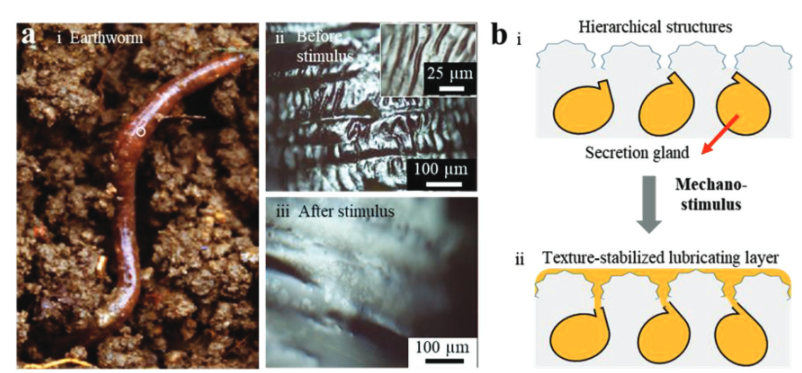
Figure 10: Earthworm prototype is shown: (a) showcasing the excreting behavior under additional stimuli, with a picture of a live earthworm breaking out from moist soil (i), followed by images of the its skin before (ii) and after (iii) a mechanical stimulus; (b) provides a detailed concept of the earthworm's surface structure and its secretory mechanism. Meanwhile, Figure 2 delves into the creation and function of gel films: (a) presents a schematic of the synthetic approach and the stimulus-responsive release mechanism; (b) displays a top-view graph of a gel film forming over different time intervals; (c) depicts the oil release process of a gel film after being washed with water and subjected to local pressure. The scale of films thickness in parts (b) and (c) measures approximately 400 µm
4. Synthetic anti-fouling surfaces
Based on those exceptional natural surfaces, various anti-fouling surfaces can be synthesized which exhibit different antifouling ways.
Antifouling surface topographies, featuring micro- to nanoscale textures, are challenging to produce using traditional manufacturing methods. Consequently, several indirect manufacturing techniques have been developed to replicate these naturally occurring topographies, categorized into four main types: lithography, chemical etching, molding, and surface corrugation (refer to Figure 11) [53].
Molding can be used indirectly to create BI topographies. Clasen and Kesel (2019) inserted microspheres into a substrate material. After solidification, this process results in a surface adorned with microdomes or exposed microparticles in part, as shown in Figure 11(b). The size of embedded microparticles crucially influences the resolution of the resulting topographies. Using nanoparticles can yield features at the nanoscale [87]. However, this technique has limitations, including a restricted selection of particle shapes—only stars, prisms, spheres, and rods—and the uncontrollable position and direction of these particles [88].
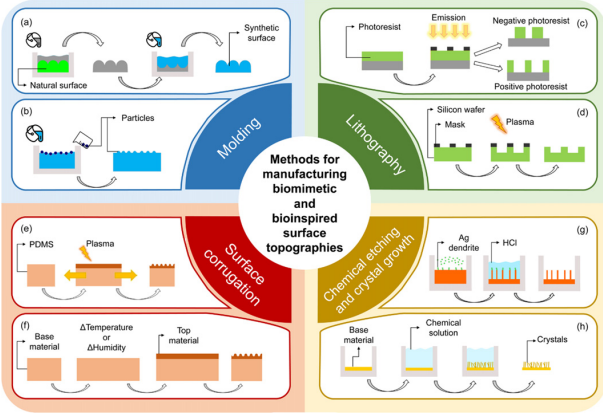
Figure 11: Methodology for creating BM/BI surface topographies is categorized into techniques of molding, lithography, surface corrugation, and chemical etching with crystal growth. In molding, there are two approaches: (a) a two-stage process that replicates the topography of a naturally existing surface and (b) using compatible particles to engineer nano and micro surface topographies. For lithography, (c) photolithography and (d) plasma lithography is utilized to fabricate BI surface textures. Surface corrugation involves creating wrinkled surfaces through (e) mechanical pre-strain or (f) strain misfit induced by changes in temperature and humidity. Lastly, (g) metal-assisted chemical etching and (h) crystal growth techniques are employed to develop nanostructures on surfaces. These methods are pivotal for advancing surface engineering by mimicking biological interfaces [53]
Photolithography is originally developed for the micro-electromechanical industry [89], which utilizes UV light or lasers along with a photomask to etch nano- and micro-patterns onto photoresist films, and it is illustrated in Figure 11(c). The primary drawback of this technique is its high cost, largely attributable to costly photomasks and sophisticated equipment required. To mitigate these costs, maskless photolithography was introduced [90], where the light beam is precisely controlled to expose specific areas of the photoresist according to the desired geometry. Various methods are used to direct the light beam, such as zone-plate array lithography, which employs a group of diffractive lenses to manage light intensity, and scanning-electron beam lithography, which uses a beam deflection system to design the patterns [91]. Unlike traditional photolithography, photoresist is not necessary for meshless photolithography, and it can achieve features smaller than 100 nm, although the results are highly dependent on the specific setup used [92]. Additionally, reactive-ion (plasma) lithography, depicted in Figure 11(d), uses a patterned mask to fabricate BI nano- and micro-structures, leveraging similar principles but with different technological applications [93].
The two methods previously discussed share a common limitation: they lack the capability to produce antifouling surfaces on a macro-scale. To address this, an alternative approach involves manufacturing surfaces with controllable wrinkles. As depicted in Figure 11(e) [94], the process begins with a polymeric material, typically a PDMS film, which is handled by pre-stretching and exposing to UV light or oxygen plasma. This exposure alters the surface of PDMS, transforming the uppermost layer into a silica-like material with strong rigidity [88]. When the pre-stretch is released, the resulting products create a wrinkled surface caused by the mechanical instability of the stiffer top layer compressing. The characteristics of these wrinkles, concerning their amplitude and wavelength, are determined by variables like irradiation time, power, and the level of pre-strain applied. Further adjustments to the wrinkled surface can be achieved by altering the temperature [95], humidity [96], or through chemical reactions [97], as shown in Figure 11(f).
Besides, crystal growth and chemical etching are also key techniques for creating bio-inspired surface topographies, particularly for nanoscale features. For instance, the method of metal-assisted chemical etching was used to produce nano-spikes with a high aspect scale in Figure 11(g) [63]. The process starts with the growth of silver dendrites on a silicon wafer. It is then immersed in hydrochloric acid for etching, where the silicon under the silver dendrites remains protected, allowing etching to occur only in exposed areas. This results in the formation of nano-spikes, the height of which depends on the duration of the etching. Subsequently, the silver dendrites are removed using a solution of ammonium hydroxide and hydrogen peroxide, revealing the silicon nano-spikes beneath. Similarly, antifouling nanostructures can be produced through crystal growth techniques, as demonstrated in Figure 11(h). Fu et al. (2018) successfully grew NiCo2O4 nanoflakes on graphite paper, which showed excellent resistance to tubeworm larval settlement. By varying the additives during synthesis, this method can also be adapted to produce NiCo2O4 nanorods and hierarchical nanostructures (G. Zhang & Lou, 2013a). Additionally, the feasibility of extending this approach to other conductive substrates has been proven (G. Zhang & Lou, 2013b).
Dynamic surfaces represent an innovative physical anti-fouling strategy, inspired by natural phenomena such as the constantly changing skin of a dolphin navigating turbulent waters. [98] This concept involves designing surfaces that can actively remove unwanted materials through mechanical action, making them especially valuable in settings where debris, ice, or biological growth frequently accumulate [48].
A practical application of this principle is seen in the design of pneumatic de-icing boots. These boots are made of rubber and fitted onto the leading edges of aircraft wings and propellers. By inflating and deflating rapidly, the boots induce mechanical stress on any ice that has formed, causing it to crack and dislodge. [99] This system is particularly beneficial for maintaining safe and efficient flight operations, as it enables the active removal of ice, thereby preserving the aircraft’s aerodynamic integrity and safety [100].
Beyond physical anti-fouling approaches, the development of chemical antifoulants presents a formidable alternative. Studies have demonstrated that certain marine organisms like corals and sponges contain bacteria that produce active organic compounds, defending against microbial encroachment [66]. For instance, metabolites secreted by bacteria from the sponge Aplysina gerardogreeni were found to suppress the development of infectant bacteria and microalgae effectively. In antifouling experiments, Sánchez-Lozano et al. [101] applied ethanol and dichloromethane extracts from assorted macroalgae combined with methanol extracts from two sponge subtypes. Their forty-day field trial revealed that coatings containing extracts from Sargassum horridum macroalgae and Haliclona caerulea sponge reduced biofouling by as much as 32%.
An additional chemical tactic involves targeting quorum sensing (QS), a crucial process for communication within bacterial colonies that influences gene expression relative to population density [99]. This bacterial interaction facilitates adhesion and biofilm development [102]. Dobretsov et al. implemented a high-throughput screening method to identify natural products that inhibit quorum sensing [103]. Of the 78 tested, about a quarter displayed quorum sensing inhibitory properties, with seven, including kojic acid, showing notable efficacy. Kojic acid proved particularly effective in lab and mesocosm studies, combating both bacterial and algal fouling. Moreover, furanones, especially when halogenated, have effectively interrupted various quorum sensing pathways, underscoring the significant role of chemical antifoulants in controlling marine biofouling [104].
Butanolide has emerged as a promising antifoulant since it is synthesized in 2009, mirroring structures found in antifouling ingredients generated by marine Streptomyces species.[102] This compound has good biodegradability and minimal toxicity to non-target species. And it can effectively prevent various fouling organisms from adhering to the function of disrupting primary energy metabolism [105]. Butanolide was recently applied to integrate into a biodegradable poly(ε-caprolactone)-based polyurethane to create an eco-friendly coating. With significant fouling prevention capabilities demonstrated, it can last for more than three months [106]. Besides, there are more improvements of butanolide coating which extended its efficacy, and corresponded fouling resistance is shown for up to six months under optimal conditions [91].
5. Limitations of current antifouling surfaces
There are many limitations of the current generation of antifouling surfaces. One of the major limitations of current anti-fouling surfaces is durability. It is a significant challenge faced by antifouling surfaces. Many coatings degrade over time due to physical wear, chemical breakdown, or UV exposure, which reduces their antifouling performance. Self-polishing copolymer (SPC) coatings [107], which slowly release biocides over time, tend to have a longer service life but still suffer from eventual degradation and loss of efficacy [19]. And polymer-based antifouling coatings, which are commonly used in the biomedical field, also face durability challenges. These coatings can be susceptible to mechanical damage and chemical breakdowns, particularly in aggressive environments such as blood or seawater. The degradation of these coatings can lead to a loss of antifouling performance, reducing their effectiveness overtime [19]. Therefore, enhancing the durability of antifouling surfaces remains a critical challenge for the development of next-generation solutions.

Figure 12: Images of marine antifouling coatings comprising stainless steel plats coating paint after immersion in the South China Sea (N 22。33I, E 114。32I) for a month. (No. 52) Control plate; (No. 45) Hydrophobic coating; and (No. 48) Hydrophilic coating [93]. Figure reproduced with permission from Progress in Organic Coatings (Elsevier)
Besides, the high cost of developing, applying, and maintaining antifouling surfaces is a significant barrier to their widespread adoption. Advanced coatings, such as those based on nanomaterials or complex surface modifications, often involve expensive materials and intricate fabrication processes that are difficult to scale up for large-scale applications [108]. For instance, superhydrophobic surfaces, which are designed to repel water and reduce biofouling, require precise micro- and nano-structuring that can be challenging to reproduce consistently on a large scale.
Another important question is that Current antifouling technologies often show limited efficacy against the full spectrum of fouling organisms. While some coatings are highly effective against microfouling (e.g., bacteria and diatoms), they may fail to prevent macrofouling by larger organisms like barnacles and mussels [20]. This selective efficacy can result in partial fouling, which still contributes to the overall negative impacts associated with biofouling. Moreover, some organisms have developed resistance to commonly used antifouling agents, further diminishing the effectiveness of current coatings [109].
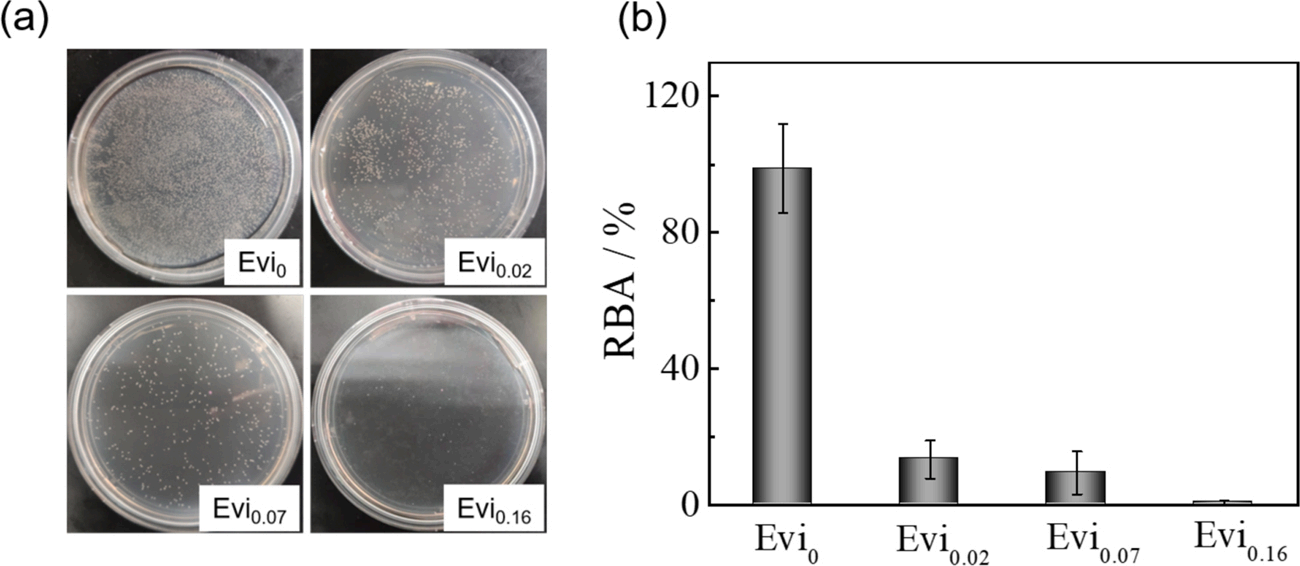
Figure 13: (a) Photographs of marine bacterial colonies of Pseudomonas sp after incubation with treated coatings containing Econea (0, 2, 7, and 16 wt%) at 30 ◦C for 24h and (b) the relative bacterial adsorption (RBA)
Additionally, they also have an impact on the environment. Many antifouling coatings, particularly those used in marine applications, rely on biocides like tributyltin (TBT) [110] and copper-based compounds to deter fouling organisms. While effective, these chemicals have been shown to leach into the water, causing significant ecological damage [19], including toxicity to non-target marine species and the disruption of aquatic ecosystems [108]. As a result, there has been a push towards developing more environmentally friendly antifouling solutions, such as non-toxic, bioinspired surfaces.
Examples of self-cleaning surfaces



Superhydrophobic lotus (N. nucifera) leaf (reproduced with permission from Bhushan et al.)


Superhydrophobic butterfly (P. lcarus) wings (adapted from W. Barthlott)



Superhydrophobic pigeon feathers (reproduced with permission from Bormashenko et al.)

Superoleophobic fish (Micropterus) scales (reproduced with permission from Liu et al.)


Low-drag shark (S. acanthias) skin (reproduced with permission from Jung & Bhushan)
Figure 14: Antifouling examples from nature using wettability properties. Water promotes self-cleaning on low adhesion superhydrophobic surfaces, as shown in the lotus, butterfly and pigeon examples. Also shown are fish scales, which are superoleophobic when submerged. Shark skin is antifoul owing to a combination of features including low drag, riblets, flexion of scales and mucous layers. (Online version in color)
However, these alternatives often suffer from reduced efficacy and durability compared to traditional biocide-based coatings [111]. As the demand for effective antifouling surfaces grows, overcoming these challenges will be crucial for the advancement of antifouling technologies that are both environmentally responsible and economically viable.
6. Strategies to minimize both soft and hard fouling contamination
Previous antifouling surfaces have only shown hope in reducing the adhesion of a single pollutant, and antifouling technology for multiple pollutants is still an urgent challenge to be solved. The key question is whether a single, durable surface that can effectively prevent these two types of fouling can be produced, and the answer lies in an interdisciplinary approach that combines physical, chemical, and biological strategies. One of the most promising ways to make this surface is through surface texture manipulation. For example, Jeremy C Thomason et al. produced a set of ceramic tiles for field research on sedimentation and biofouling and proved their durability and robustness through the design of ceramic tile surface texture [21] Scardino et al. minimized the attachment points of fouling organisms by introducing micron or nanometer roughness on the surface. [112] Then, inspired by natural structures such as lotus leaves, Vizhi et al. and Zhou et al. designed superhydrophobic surfaces through special nanoengineering techniques with low surface roughness and surface energy, which can make both soft and hard fouling organisms difficult to stand on.
In addition to physical structures, advanced chemical coatings play a critical role in fouling prevention. Materials like fluoro group polymers [113] or silicone-based coatings [114] are effective because they lower the surface energy, making it difficult for fouling organisms to adhere. Furthermore, antimicrobial agents can be embedded into the surface, actively preventing soft fouling by killing bacteria and inhibiting biofilm development. [22]
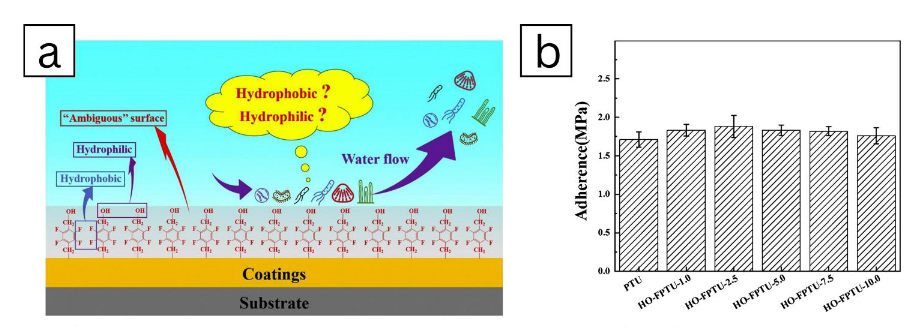
Figure 15: Utilize fluoro polymer coating as a technique of advanced chemical coating
Recently, there has been a novel technique for anti-fouling whose technique has never been demonstrated. Zahra Azimi Dijvejin and his team have demonstrated that the underside of the kirigami sheet is superhydrophobic (SH) (i.e. inverted) and, when suspended over the substrate, significantly reduces the adhesion of many different solid fouling agents, including ice, wax, dry sludge and biofouling mimics (pseudo barriers) such as epoxies and acrylic tapes. [115] Kirigami sheet is an ancient paper-cutting technique in Japan, which could adjust the geometric structures dynamically [23].
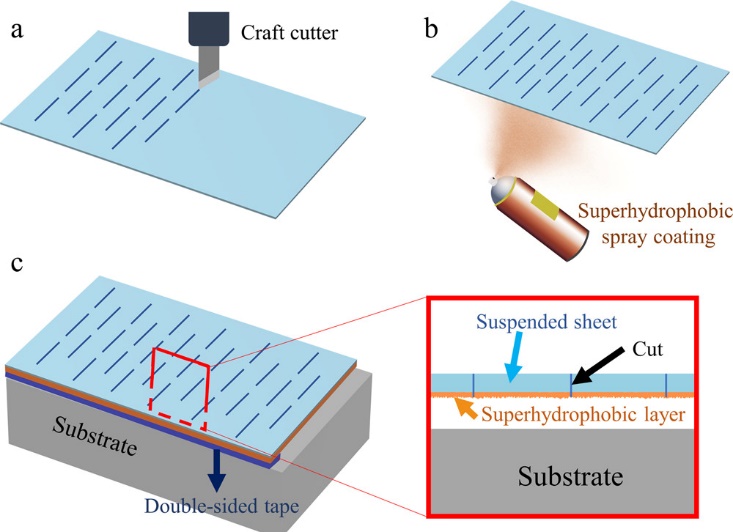
Figure 16: Schematic of SKINS fabrication
Achieving a single pollution system is promising, but multi-pollutant antifouling technology remains an elusive problem in many industries.[11] It is still challenging to achieve a low-cost, easy-to-apply, efficient and durable antifouling coating, but it is very promising to apply synergistic advantages, such as the research described above, to combine several physical and chemical antifouling strategies into a versatile coating.
7. Conclusion
Overall, bio-inspired antifouling surfaces show considerable promise for the future. With the introduction of new environmental policies, the development of these surfaces is increasingly essential. Such innovations can draw inspiration not only from micro- and nano-scale topographies, like those of mangrove trees and seeds, but also from natural antifoulants produced by bacteria in invertebrates such as sponges and corals. Additionally, surfaces exhibiting dynamic properties, such as shedding effects and instability, can offer effective deicing capabilities. Beyond these inherent properties, some natural surfaces are enhanced with mucus-like hydrogels or zwitterionic coatings. Combining these intrinsic properties with external antifouling coatings can lead to surfaces with even more exceptional characteristics. Despite the advances, current antifouling technologies still face limitations, particularly regarding cost and durability. Nevertheless, integrating both physical and chemical antifouling strategies into a versatile coating holds the potential to overcome these challenges and realize more effective solutions.



