1. Introduction
Not long ago, significant energy efficiency and effectiveness were realized in electric vehicles and portable devices by the development of lithium-ion batteries. While battery studies often lay more emphasis on capacity, charge/discharge rates, cycle life, and safety performance, the SEI relies on electrochemical phenomena to gain insights into the SEI's characteristics. The research work covers examining the SEI layers. The solid electrolyte interface (SEI) is a thin film case that is observed at the surface of an electrode and functions as a key area of study. Introduced by Peled in 1979, the SEI layer is critical for stabilizing electrode performance in lithium-ion batteries (LIBs) [4].
LIBs being equipped with a "sandwich" structure that includes a positive electrode, a negative electrode, a separator, an electrolyte, and current collector that hold all these together. The SEI film, as shown in Figure 1, a diaphanous nanolayer formed by the reaction between the carbonate of the electrolyte and the oxide of electrodes comprises the periphery of the sodium battery, an organic lithium compounds layer, and an inorganic lithium compounds parts. This film is both ion conductive and dielectric, then lithium ions will conduct in from the outside, and the characteristics will assist in the stabilization and enhancement of the battery performances. Nevertheless, lithium metal anodes are degraded; this leads to an organic layer of the SEI to be formed on the surfaces of the lithium metal, which causes short circuits. The main objective of a research team is to overcome the above-mentioned difficulties; the team has selected graphite material instead of lithium metal.
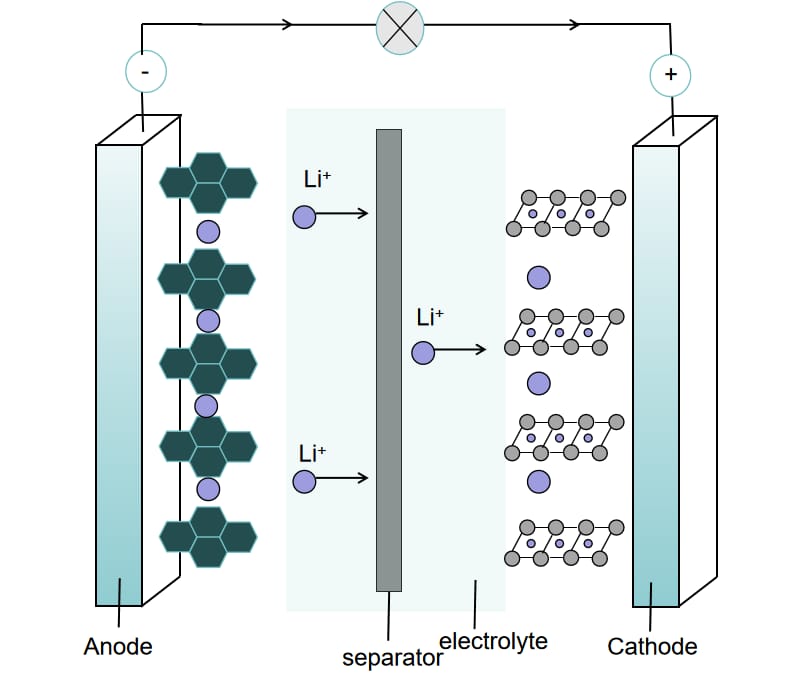
Figure 1: Internal diagram of SEI film of lithium ion battery
2. Background on Lithium-Ion Batteries
Owing to its layered structure and a redox potential close to Li⁺/Li, graphite is not deposited into lithium metal, and the dendrites will not be formed on the anode, making it a safe anode choice. Furthermore, it is cheap and widely available. Hence, this is why it is the most used technology for commercial batteries.
In 1990, Aldoprehid was suggested to prevent the interference of solvated species and stabilize the SEI film by using vinyl carbonate [5]. A stable SEI layer boosts the battery's electrical qualities and stability; its energy density, charge/discharge rates, cycling performance, and safety. In addition, it reduces manufacturing costs, which are an important aspect as well.
Deep pursuit of the heating and forming of SEI at working conditions is important in improving the operation and safety of the battery. The investigation is complicated due to many aspects that affect SEI formation, such as materials properties of the electrodes, electrolyte compositions, graphite dust morphology, electrochemical parameter, and battery temperature. Appreciate the influence of these factors as well as the charging/discharging functions of Li-ion are critical in the development of the next generation batteries.
3. SEI Membrane Development Mechanism
3.1. Formation Mechanism of SEI Membranes
The solid electrolyte interface (SEI) membrane, which has an important role in the performance and lifetime of lithium-ion batteries, is a critical element. Therefore, gaining the knowledge concerning the SEI membrane components is the most important factor to guarantee the future development of batteries. By doing so, the role of each of the components of the SEI membrane system can help us to improve the design of SEI membrane and identify the optimized structure of SEI membrane to maximize the battery capacity and safety.
In developed literature, SEI membranes are majorly given as polymers, inorganic salts, and electrolyte solutions, which is illustrated in Figure 2. Within the SEI membrane, the polymer plays a key role in ion diffusion, stabilizing the electrode active materials, and further providing stress accommodation to the active material parts. Inorganic salt compounds do improve the membrane’s conductivity and stability. The choice of the electrolyte solvent affects the mechanical property and chemical characteristics of the sol-gels. The electrolyte constitutes lithium salt - usually lithium hexafluorophosphate [17] - and carbonate solvent [19], as well as some functional additives. Regardless of the electrolyte nature and their nucleation stage, which are similar, the SEI composition may, nevertheless, vary.
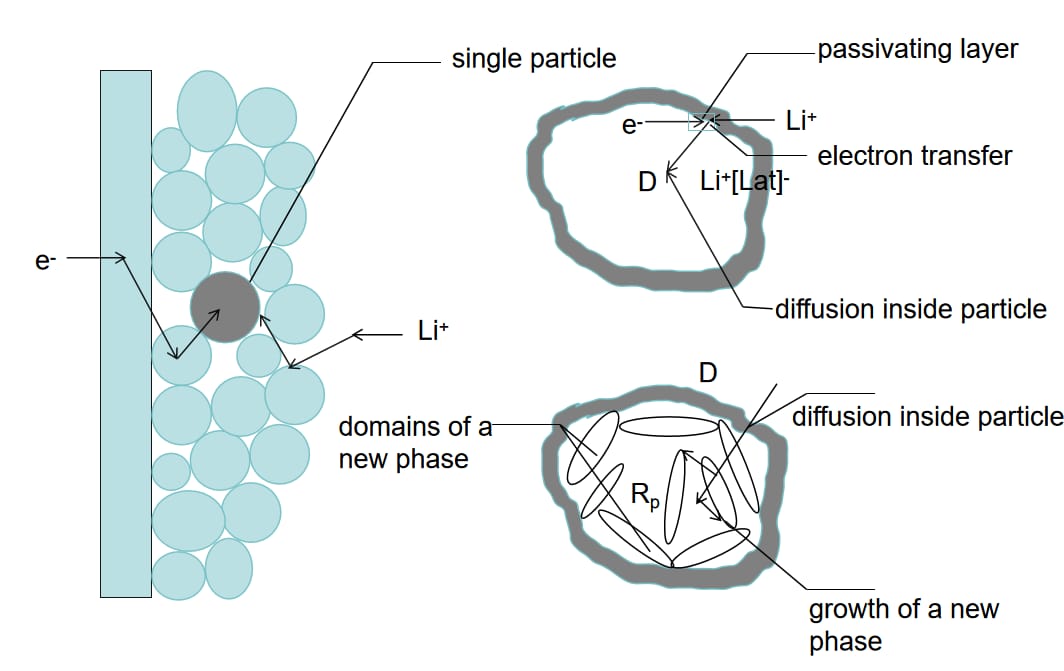
Figure 2: Illustration of lithium-ion diffusion, electron transfer, and phase formation within a single particle in a lithium-ion battery, highlighting the impact on SEI membrane structure and stability.
Goodenough [6] suggested that the anode material surface potential is affected by the charge cycle at first and thus is lower than it is supposed to be. When the anode is charged, lithium cations and hydroxides are the result, which react to produce battery capacity. For SEI films of graphite anodes in lithium-ion batteries with a vinyl carbonate and propylene carbonate-based lithium salt solution, we can group them into three stages: (Figure 3 echoes this.)
(1) Initial stage (≈2.0 V): Water electrolysis, lithium ions solvation, and the hydroxide matter to produce LiOH.
(2) Intermediate Stage (2.0-2.5 V): Initial degradation of the vinyl carbonate (an electrolyte precursor) to form lithium carbonate, eventually leading to the formation of a thin layer (nucleus) on the anode.
(3) Advanced Stage (above 2.5 V): PC and PP reactions, the production of many decomposition products including soluble deposits, insoluble deposits, and gaseous materials. Gases lead to swelling, and STAY in electrolyte as thin electrolyte film. Thus, only insoluble deposits are the film ingredient.
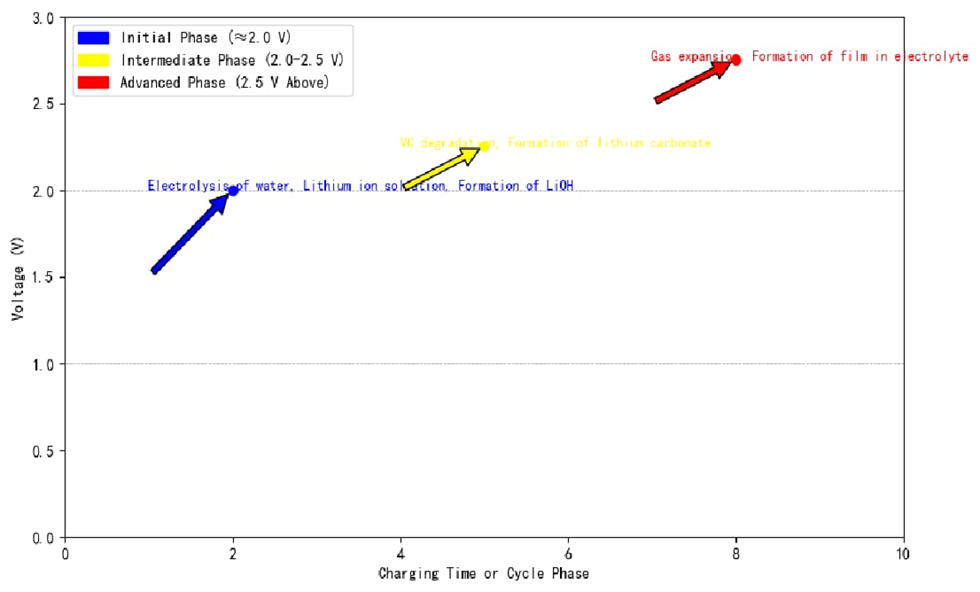
Figure 3: Stages of SEI membrane formation in graphite anodes at different voltage levels, highlighting chemical reactions.
3.2. Composition of SEI Membranes
The chemistry and morphology of SEI film alter with the positively and negatively charged current electrode materials. Consequently, the different types of materials release different gases and products during charging/discharging cycles, which influence the SEI composition. Likewise, interstitial ions intercalated and oxidized positive electrodes release oxides, graphite anodes expand and reduce due to changes made in the inner structure. As the carbon surface of the graphite anode, reactive location, and electrolyte constitute the SEI film. Solubility of electrolytes, ionic conductance, and chemical stability are the chain reaction controlling the SEI film formation rate and even its quality.
It is being mentioned in the study that the SEI film also, which is subjected to irrevocable changes, is altered. The result is a film which detaches and thickens, creating structural damage. For silicon anodes, the amount of silicon conduction cells change with charger cycles (up to 300%). This leads to SEI layer splitting and wrapping in response to direct contact with the electrolyte. [22]. Such cyclic deterioration can restrict charging works and add the capacity impairment. The nature of the electrolyte remains a crucial factor affecting SEI film generation and stability.
3.3. Analytical Techniques for SEI Membranes:
X-ray Photoelectron Spectroscopy (XPS): Through XPS, the SEI membrane surface elemental composition and degree of the chemical states status can be analyzed, showing the change of carbon, oxygen, and sulfur content over the time [9].
Raman Spectroscopy: This method gives insights into the structural and the chemical bonding change of the SEI layer; consequently, it denotes possible loosening of the membrane structure and deterioration of the SEI membrane shielding itself [8][12].
4. Lithium-Ion Battery Aging
It is inevitable that lithium-ion batteries will be subject to aging with the subsequent use, which in turn will depend on the charge/discharge cycles frequency as well as on the intervals between the usages, and the ambient temperature levels [27][28]. Aging is the term applied to the process of battery capacity decrease and increase in impedance, which leads to lower electrochemical performance.
4.1. Key Factors Contributing to Capacity Decline:
(1) SEI Film Extension: A thick or uneven SEI membrane can lead to lack of ion transfer and increase of internal impedance [4][5].
(2) Electrolyte Decomposition: An electrolyte can degrade, resulting in formation of degradation products that manifest in diminished battery performance [18][19].
(3) Self-Discharge: The self-discharge of lithium-ion batteries is often more perturbed with age [25].
(4) Extracting of Electrode Matter: The active compound reduction, thus decreasing the capacity of the cells [21][22].
(5) Collector Corrosion: Loss of metals in current collectibles changes impedance and lowers efficiency [26].
While the aging course, several chemical reactions take place in the energized cell, resulting in the distortion of its structure and the appearance of damaging products. The seered materials are oxides, carbonates, and organic compounds as presented in [26].
4.2. Types of Aging:
Calendar Aging: Capacity of battery which decreases while the battery is in storage without having the charge or discharge cycles [27].
Cyclic Aging: Memory refers to the failure which is also irreversible during repeated charge/discharge cycles [28].
Capacity is one of the most important performance parameters of the lithium-ion battery and indicates the amount of electricity that the battery can deliver at a given rate. The capacity fading is a very profound manifestation of the battery aging, and through capacity decay pattern study, the internal aging mechanisms of the battery can be identified.
Influence of SEI Layering: The SEI layer development is in perpetual creation and destruction in the process of charge/discharge cycles. To begin with, the rate of oxygen-containing functional groups is lower, while that of graphite increases, which results unevenness and thickness of the SEI layer over time [4][5]. Thus, higher thermal internal resistance, lower capacity, and safety issues are expected. The dielectric properties change with respect to the cycle number, temperature, and charge/ discharge levels [6][7]. Aging SEI could display natural defects like cracks and increased permeation, which facilitate corrosion, and degradation to a greater extent. High temperature promotes membrane instability and thus fast aging of the SEI membrane [23].
4.3. Other Causes of Aging:
Solvent Co-embedding: Through gas evolution, particle cracking, graphite flaking, and losing activity of material may occur [24].
Collector Corrosion: Over-voltage caused inhomogeneous distribution of current and potential that leads to an increased resistivity. It is better to do pre-treatment of collectors which can be a solution to this issue [26].
Lithium Precipitation: High and low temperatures, mainly at the end of the charging period, lead to lithium precipitation that can raise these rates. Lithium precipitation is responsible for reducing the internal porosity of negative electrodes, while increasing the electrolyte potential gradient, and hence accelerating the battery aging [23].
4.4. Impact of Structural Changes:
Cathode Material Changes: Structural alterations and stress accumulation in active materials of cathodes can initiate microcracks and phase excess, sowing the age related further deterioration [23].
Graphite Anode Changes: All in all, there are minor changes in the material of the graphite anode. However, the interface between the anode/electrolyte has a huge effect on the performance of the battery [21][22].
Monitoring and Controlling Aging: Aging can be tracked by means of electrochemical methods and thus controlled through the use of (EIS) [10] and state of charge (SOC), (SOH), Direct Current Resistance (DCR), and the potential changes. These processes are also effective to assess pulse and non-pulse battery behavior, which extends battery life and promotes improvements in battery technology.
5. Characterization Techniques
5.1. Chemical Composition Analysis of SEI Membranes
Chemical Composition Analysis of SEI Membranes Studying the chemical structure of the solid electrolyte interphase (SEI) layer is a vital component of the lithium-ion battery research field. The analysis, focusing on the SEI membrane's structural, chemical, and performance Microscopy (SRM) provides inputs to improve the battery performance and prolong its operational life. The physical properties and performance of the SEI membranes are closely bound to their chemical composition, and this is as a result, determining the need to analyze the membranes.
There are numerous techniques, many of which are called characterization techniques CV, EIS and all those things that are employed to build the methods from what is happening on the surface of the membranes.
5.2. Electrochemical Methods
Electrochemical Methods: Electrochemical methods, mainly cyclic voltammetry (CV) [11] and electrochemical impedance spectroscopy (EIS) [10], are used for knowing the SEI membranes characteristics through a particular electrochemical parameter of the electrodes. In EIS study, the performance of the SEI membrane is evaluated. This technique is used to investigate the charge and discharge performance of a single cell or a battery pack and its lifetime by monitoring the SEI layer impedance. The AC impedance study, in its turn, will visualize the processes of SEI layer formation and degradation as they correspond to the impedance increases and decreases respectively. The EIS of the electrode at these diverse storage conditions and numbers of storage cycles can reflect the secrets of newly formed, deteriorating, or completely disappeared SEI films.
A slow sweeping of CV method (less than 1 mV/s) of graphite electrodes that are initially not charged and discharged, and ultimately followed by a potentiometric measure of the first and second scans, which produced a reduction peak around 0.7 V, but then that peak completely disappeared in the second scan. However, this loss of Dopamine current was used as the peak of the present to the formation of the SEI membrane. The CV test discloses the electrochemical stability and degradation mode of the SEI membrane, giving a reference and good test case for designing the cell design. The EIS technique is capable of ziching deeply into the ion transport characteristics and the impedance aspects of the SEI membrane. In this case, we will evaluate the charging and discharging of the battery as well as their cycle life span. As a result, given the number of methods for characterization of the chemistry of SEI membranes, which are known at present, there are a great number of tools (spectroscopic and chromatographic) for studying SEIs and characterizing their chemical composition.
5.3. Spectroscopic and Chromatographic Techniques.
This study dealt with the application of both spectroscopic and chromatographic analyses for the assessment of the kind and amount of the SEI constituents. Two examples of widely applied techniques in the study of SEI, as well as the methods used for their analysis, is FTIR spectroscopy [12] and Raman spectroscopy [8]. FTIR is utilized to detect functional groups within the SEI membrane. It helps to specify the nature of the bonding and structure of the molecules that are formed via the electrolyte decomposition and interaction with the electrode material.
Ogumi's [7] chromatographic mass spectroscopy (CS-MS) analysis of graphite electrodes surface of charged batteries, in addition to the known ROCOOLi and Li₂CO₃, also detected various ethers of diols and low molecular weight polymers (PEOs) containing vinyl-oxygen units, which, in turn, proved that these oligo's find their way into SEI membranes. This clearly confirms that oligomers - a polymer deriving from the original molecule - are present in those membranes. Currently, due to the improvement of electron microscopy technology, one can directly use data deduced from electrical and optical measurements to interpret the microstructure and the property of the SEI membranes or directly - observe the state and structure of the SEI membranes grown on the surface of electrodes. From tens of thousands to millions times of magnifying electron, we gradually apply those methods to the study of SEI membranes. Giving rise to more evidence for SEI membranes. In this regard, the most ultimate tools include transmission electron microscopy (TEM), atomic force microscopy (AFM), and scanning probe microscopy (SPM) (with millions of times magnifications). Besides, various analytical techniques usually result in the detail and comprehensive knowledge of the SEI membrane's chemical composition and behavior. Such information is valuable for improving conductivity of systems, improving delivery abilities, and prolonging life.
6. Conclusion
The investigation of the SEI membranes is the most emphasized area of research focusing on the growing popularity of lithium batteries, which we hold today. Understanding both the mechanism of the formation of SEI membranes and the electrochemical properties of it is theoretically very significant, as it helps to guide the determination of the capacity fading mechanism of lithium batteries and the design of advanced materials of the batteries efficiently. The focus of the SEI membrane study will be much the same with the continuing development of lithium-ion batteries, the primary direction of this. The SEI membrane is constitutive of the nanoscale, and because of its nature, the figure of the SEI membrane and the list of compounds might be affected constantly as the cold and the hot, as well as the non-insitu traditional techniques. Because of this, strategically developing new in-situ techniques enabling precise monitoring of dynamics SEI constituents under real-time conditions of lithium-ion battery should be a plan.



